A viral suppressor of gene silencing in plants
- PMID: 9789044
- PMCID: PMC23715
- DOI: 10.1073/pnas.95.22.13079
A viral suppressor of gene silencing in plants
Abstract
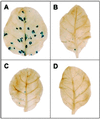
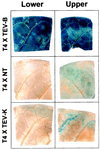
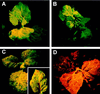
Gene silencing is an important but little understood regulatory mechanism in plants. Here we report that a viral sequence, initially identified as a mediator of synergistic viral disease, acts to suppress the establishment of both transgene-induced and virus-induced posttranscriptional gene silencing. The viral suppressor of silencing comprises the 5'-proximal region of the tobacco etch potyviral genomic RNA encoding P1, helper component-proteinase (HC-Pro) and a small part of P3, and is termed the P1/HC-Pro sequence. A reversal of silencing assay was used to assess the effect of the P1/HC-Pro sequence on transgenic tobacco plants (line T4) that are posttranscriptionally silenced for the uidA reporter gene. Silencing was lifted in offspring of T4 crosses with four independent transgenic lines expressing P1/HC-Pro, but not in offspring of control crosses. Viral vectors were used to assess the effect of P1/HC-Pro expression on virus-induced gene silencing (VIGS). The ability of a potato virus X vector expressing green fluorescent protein to induce silencing of a green fluorescent protein transgene was eliminated or greatly reduced when P1/HC-Pro was expressed from the same vector or from coinfecting potato virus X vectors. Expression of the HC-Pro coding sequence alone was sufficient to suppress virus-induced gene silencing, and the HC-Pro protein product was required for the suppression. This discovery points to the role of gene silencing as a natural antiviral defense system in plants and offers different approaches to elucidate the molecular basis of gene silencing.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

