Patch-clamp and amperometric recordings from norepinephrine transporters: channel activity and voltage-dependent uptake
- PMID: 9789076
- PMCID: PMC23775
- DOI: 10.1073/pnas.95.22.13260
Patch-clamp and amperometric recordings from norepinephrine transporters: channel activity and voltage-dependent uptake
Abstract
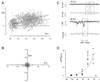
Transporters for the biogenic amines dopamine, norepinephrine, epinephrine and serotonin are largely responsible for transmitter inactivation after release. They also serve as high-affinity targets for a number of clinically relevant psychoactive agents, including antidepressants, cocaine, and amphetamines. Despite their prominent role in neurotransmitter inactivation and drug responses, we lack a clear understanding of the permeation pathway or regulation mechanisms at the single transporter level. The resolution of radiotracer-based flux techniques limits the opportunities to dissect these problems. Here we combine patch-clamp recording techniques with microamperometry to record the transporter-mediated flux of norepinephrine across isolated membrane patches. These data reveal voltage-dependent norepinephrine flux that correlates temporally with antidepressant-sensitive transporter currents in the same patch. Furthermore, we resolve unitary flux events linked with bursts of transporter channel openings. These findings indicate that norepinephrine transporters are capable of transporting neurotransmitter across the membrane in discrete shots containing hundreds of molecules. Amperometry is used widely to study neurotransmitter distribution and kinetics in the nervous system and to detect transmitter release during vesicular exocytosis. Of interest regarding the present application is the use of amperometry on inside-out patches with synchronous recording of flux and current. Thus, our results further demonstrate a powerful method to assess transporter function and regulation.
Figures



Comment in
-
Neurotransmitter transport: models in flux.Proc Natl Acad Sci U S A. 1998 Oct 27;95(22):12737-8. doi: 10.1073/pnas.95.22.12737. Proc Natl Acad Sci U S A. 1998. PMID: 9788979 Free PMC article. Review. No abstract available.
References
-
- Trendelenburg T. Trends Pharmacol Sci. 1991;12:334–337. - PubMed
-
- Giros B, Caron M G. Trends Pharmacol Sci. 1993;14:43–49. - PubMed
-
- Amara S, Kuhar M J. Annu Rev Neurosci. 1993;16:73–93. - PubMed
-
- Blakely R D, DeFelice L J, Hartzell H C. J Exp Biol. 1994;196:263–281. - PubMed
-
- Lester H A, Mager S, Quick M W, Corey J L. Annu Rev Pharmacol Toxicol. 1994;34:219–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

