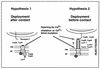
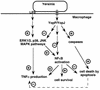
The Yersinia deadly kiss
- PMID: 9791096
- PMCID: PMC107605
- DOI: 10.1128/JB.180.21.5495-5504.1998
The Yersinia deadly kiss
Figures


References
-
- Allaoui A, Schulte R, Cornelis G R. Mutational analysis of the Yersinia enterocolitica virC operon: characterization of yscE, F, G, I, J, K required for Yop secretion and yscH encoding YopR. Mol Microbiol. 1995;18:343–355. - PubMed
-
- Anderson D M, Schneewind O. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science. 1997;278:1140–1143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

