Domain analysis of the FliM protein of Escherichia coli
- PMID: 9791106
- PMCID: PMC107615
- DOI: 10.1128/JB.180.21.5580-5590.1998
Domain analysis of the FliM protein of Escherichia coli
Abstract
The FliM protein of Escherichia coli is required for the assembly and function of flagella. Genetic analyses and binding studies have shown that FliM interacts with several other flagellar proteins, including FliN, FliG, phosphorylated CheY, other copies of FliM, and possibly MotA and FliF. Here, we examine the effects of a set of linker insertions and partial deletions in FliM on its binding to FliN, FliG, CheY, and phospho-CheY and on its functions in flagellar assembly and rotation. The results suggest that FliM is organized into multiple domains. A C-terminal domain of about 90 residues binds to FliN in coprecipitation experiments, is most stable when coexpressed with FliN, and has some sequence similarity to FliN. This C-terminal domain is joined to the rest of FliM by a segment (residues 237 to 247) that is poorly conserved, tolerates linker insertion, and may be an interdomain linker. Binding to FliG occurs through multiple segments of FliM, some in the C-terminal domain and others in an N-terminal domain of 144 residues. Binding of FliM to CheY and phospho-CheY was complex. In coprecipitation experiments using purified FliM, the protein bound weakly to unphosphorylated CheY and more strongly to phospho-CheY, in agreement with previous reports. By contrast, in experiments using FliM in fresh cell lysates, the protein bound to unphosphorylated CheY about as well as to phospho-CheY. Determinants for binding CheY occur both near the N terminus of FliM, which appears most important for binding to the phosphorylated protein, and in the C-terminal domain, which binds more strongly to unphosphorylated CheY. Several different deletions and linker insertions in FliM enhanced its binding to phospho-CheY in coprecipitation experiments with protein from cell lysates. This suggests that determinants for binding phospho-CheY may be partly masked in the FliM protein as it exists in the cytoplasm. A model is proposed for the arrangement and function of FliM domains in the flagellar motor.
Figures
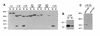





References
-
- Bischoff D S, Ordal G W. Identification and characterization of FliY, a novel component of the Bacillus subtilis flagellar switch complex. Mol Microbiol. 1992;6:2715–2723. - PubMed
-
- Bren A, Eisenbach M. The N-terminus of the flagellar switch protein, FliM, is the binding domain of the chemotactic response regulator, CheY. J Mol Biol. 1998;278:507–514. - PubMed
-
- Francis N R, Sosinsky G E, Thomas D, DeRosier D J. Isolation, characterization and structure of bacterial flagellar motors containing the switch complex. J Mol Biol. 1994;235:1261–1270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

