Reporting on quality of life in randomised controlled trials: bibliographic study
- PMID: 9794853
- PMCID: PMC28701
- DOI: 10.1136/bmj.317.7167.1191
Reporting on quality of life in randomised controlled trials: bibliographic study
Erratum in
- BMJ 1998 Nov 14;317(7169):1353
Abstract
Objectives: To examine the frequency and quality of reporting on quality of life in randomised controlled trials.
Design: Search of the Cochrane Controlled Trials Register 1980 to 1997 to identify trials from all disciplines, from oncology, and from cardiovascular medicine that reported on quality of life. Assessment of abstracts from articles published from 1993 to 1996. Assessment of a sample of full reports with a standardised instrument.
Main outcome measures: Prevalence of reporting on quality of life. Conditions and interventions studied in trials reporting on quality of life. Quality of reporting on quality of life.
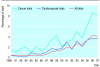
Results: During 1980-97 reporting on quality of life increased from 0.63% to 4.2% for trials from all disciplines, from 1.5% to 8.2% for cancer trials, and from 0.34% to 3.6% for cardiovascular trials. Of 364 abstracts, 65% reported on drug interventions. Of a sample of 67 full reports, authors of 48 (72%) used 62 established quality of life instruments. In 15 reports (22%) authors developed their own measures, and in 2 (3%) methods were unclear. Response rates were given in 38 (57%), and complete reporting on all items and scales occurred in 31 (46%).
Conclusions: Less than 5% of all randomised controlled trials reported on quality of life, and this proportion was below 10% even for cancer trials. A plethora of instruments was used in different studies, and the reporting of methods and results was often inadequate. Standards for the measurement and reporting of quality of life in clinical trials research need to be developed.
Figures
Comment in
-
Reporting on quality of life in RCTs. CONSORT guidelines should be expanded.BMJ. 1999 Apr 24;318(7191):1142. BMJ. 1999. PMID: 10213745 Free PMC article. No abstract available.
-
Reporting on quality of life in RCTs. Authors are creating database of quality of life questionnaires.BMJ. 1999 Apr 24;318(7191):1142. BMJ. 1999. PMID: 10366269 No abstract available.
References
-
- Patrick DL, Bergner M. Measurement of health status in the 1990s. Ann Rev Public Health. 1990;11:165–183. - PubMed
-
- Gill TM, Feinstein AR. A critical appraisal of the quality of quality-of-life measurements. JAMA. 1994;272:619–626. - PubMed
-
- Guyatt G, Feeny D, Patrick D. Issues in quality of life measurement in clinical trials. Control Clin Trials. 1991;12:81–90S. - PubMed
-
- Fallowfield L. Quality of quality of life data. Lancet. 1996;348:421. - PubMed
-
- Naughton MJ, Shumaker SA. Assessment of health-related quality of life. In: Furberg CD, DeMets DL, editors. Fundamentals of clinical trials. 3rd ed. St Louis: Mosby Press; 1996.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources