Pharmacokinetic interaction between ritonavir and indinavir in healthy volunteers
- PMID: 9797204
- PMCID: PMC105944
- DOI: 10.1128/AAC.42.11.2784
Pharmacokinetic interaction between ritonavir and indinavir in healthy volunteers
Abstract
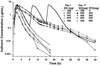
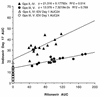
The pharmacokinetic interaction between indinavir and ritonavir was evaluated in five groups of healthy adult volunteers to explore the potential for twice-daily (b.i.d.) dosing of this combination. All subjects received 800 mg of indinavir every 8 h (q8h) on day 2. In addition, subjects in group I received one dose of 800 mg of indinavir on day 1 and 800 mg of indinavir q8h on day 17. Subjects in Groups II and IV each received one dose of 600 mg of indinavir on days 1 and 17, and subjects in groups III and V each received one dose of 400 mg of indinavir on days 1 and 17. During days 3 to 17, ritonavir placebo or ritonavir at 200, 300, 300, or 400 mg q12h was given to groups I, II, III, IV, and V, respectively. Ritonavir at steady state probably inhibited the cytochrome P-450 3A metabolism of indinavir and substantially increased plasma indinavir concentrations, with the area under the plasma concentration-time curve (AUC) increasing up to 475% and the peak concentration in serum (Cmax) increasing up to 110%. The Cmax/trough concentration ratio decreased from 50 in standard q8h regimens to less than 14 when indinavir was administered with ritonavir. For a constant indinavir dose, an increase in the ritonavir dose yielded similar indinavir AUCs, Cmaxs, and concentrations at 12 h (C12s). For a constant ritonavir dose, an increase in the indinavir dose resulted in approximately proportional increases in the indinavir AUC, less than proportional increases in Cmax, and slightly more than proportional increases in C12. Ritonavir reduced between-subject variability in the indinavir AUC and trough concentrations and did not affect indinavir renal clearance. With the altered pharmacokinetic profile, indinavir likely could be given as a b.i.d. combination regimen with ritonavir. This could potentially improve patient compliance and thereby reduce treatment failures.
Figures
References
-
- Abbott Laboratories. Norvir™ product information. North Chicago, Ill: Abbott Laboratories; 1998.
-
- Acosta E P, Henry K, Weller D, Page L M, Bacon L, Rhame F, Gilson I, Rosenstein H, Schacker T, Fletcher C V. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Indinavir pharmacokinetics and relationships between exposures and antiviral effect, abstr. A-15; p. 3.
-
- Balani S K, Woolf E J, Hoagland V L, Sturgill M G, Deutsch P J, Yeh K C, Lin J H. Disposition of indinavir, a potent HIV-1 protease inhibitor, after a oral dose in humans. Drug Metab Dispos. 1996;24:1389–1394. - PubMed
-
- Bjornsson T, Chiou R, Deutsch P, Haddix H, Hoagland V, Justice S, Nessly M, Pomerantz R, Saag M, Squires K, Teppler H, Waldman S, Woolf E, Yeh K C. Pharmacokinetics of indinavir. Pharm Res. 1996;13:S485.
-
- Bruce R G, Munch L C, Hoven A D, Jerauld R S, Greenburg R, Porter W H, Rutter P W. Urolithiasis associated with the protease inhibitor indinavir. Urology. 1997;50:513–518. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

