Comparison of the thermostability properties of three acid phosphatases from molds: Aspergillus fumigatus phytase, A. niger phytase, and A. niger PH 2.5 acid phosphatase
- PMID: 9797305
- PMCID: PMC106667
- DOI: 10.1128/AEM.64.11.4446-4451.1998
Comparison of the thermostability properties of three acid phosphatases from molds: Aspergillus fumigatus phytase, A. niger phytase, and A. niger PH 2.5 acid phosphatase
Abstract
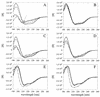
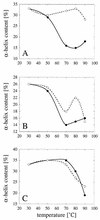
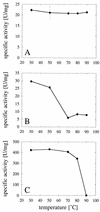
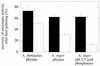
Enzymes that are used as animal feed supplements should be able to withstand temperatures of 60 to 90 degrees C, which may be reached during the feed pelleting process. The thermostability properties of three histidine acid phosphatases, Aspergillus fumigatus phytase, Aspergillus niger phytase, and A. niger optimum pH 2.5 acid phosphatase, were investigated by measuring circular dichroism, fluorescence, and enzymatic activity. The phytases of A. fumigatus and A. niger were both denatured at temperatures between 50 and 70 degrees C. After heat denaturation at temperatures up to 90 degrees C, A. fumigatus phytase refolded completely into a nativelike, fully active conformation, while in the case of A. niger phytase exposure to 55 to 90 degrees C was associated with an irreversible conformational change and with losses in enzymatic activity of 70 to 80%. In contrast to these two phytases, A. niger pH 2.5 acid phosphatase displayed considerably higher thermostability; denaturation, conformational changes, and irreversible inactivation were observed only at temperatures of >/=80 degrees C. In feed pelleting experiments performed at 75 degrees C, the recoveries of the enzymatic activities of the three acid phosphatases were similar (63 to 73%). At 85 degrees C, however, the recovery of enzymatic activity was considerably higher for A. fumigatus phytase (51%) than for A. niger phytase (31%) or pH 2.5 acid phosphatase (14%). These findings confirm that A. niger pH 2.5 acid phosphatase is irreversibly inactivated at temperatures above 80 degrees C and that the capacity of A. fumigatus phytase to refold properly after heat denaturation may favorably affect its pelleting stability.
Figures



References
-
- Chen G C, Yang J T. Two-point calibration of circular dichrometer with d-10-camphorsulfonic acid. Anal Lett. 1977;10:1195–1207.
-
- Delboni L F, Mande S C, Rentier-Delrue F, Mainfroid V, Turley S, Vellieux F M D, Martial J A, Hol W G J. Crystal structure of recombinant triosephosphate isomerase from Bacillus stearothermophilus. An analysis of potential thermostability factors in six isomerases with known three-dimensional structures points to the importance of hydrophobic interactions. Protein Sci. 1995;4:2594–2604. - PMC - PubMed
-
- Jobin Yvon. Instrument manual. Longjumeau, France: Jobin Yvon; 1990.
-
- Kawamura S, Kakuta Y, Tanaka I, Hikichi K, Kuhara S, Yamasaki N, Kimura M. Glycine-15 in the bend between two α-helices can explain the thermostability of DNA binding protein HU from Bacillus stearothermophilus. Biochemistry. 1996;35:1195–1200. - PubMed
-
- Kostrewa, D. Unpublished data.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

