Humic acid reduction by propionibacterium freudenreichii and other fermenting bacteria
- PMID: 9797315
- PMCID: PMC106677
- DOI: 10.1128/AEM.64.11.4507-4512.1998
Humic acid reduction by propionibacterium freudenreichii and other fermenting bacteria
Abstract
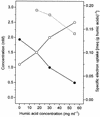
Iron-reducing bacteria have been reported to reduce humic acids and low-molecular-weight quinones with electrons from acetate or hydrogen oxidation. Due to the rapid chemical reaction of amorphous ferric iron with the reduced reaction products, humic acids and low-molecular-weight redox mediators may play an important role in biological iron reduction. Since many anaerobic bacteria that are not able to reduce amorphous ferric iron directly are known to transfer electrons to other external acceptors, such as ferricyanide, 2,6-anthraquinone disulfonate (AQDS), or molecular oxygen, we tested several physiologically different species of fermenting bacteria to determine their abilities to reduce humic acids. Propionibacterium freudenreichii, Lactococcus lactis, and Enterococcus cecorum all shifted their fermentation patterns towards more oxidized products when humic acids were present; P. freudenreichii even oxidized propionate to acetate under these conditions. When amorphous ferric iron was added to reoxidize the electron acceptor, humic acids were found to be equally effective when they were added in substoichiometric amounts. These findings indicate that in addition to iron-reducing bacteria, fermenting bacteria are also capable of channeling electrons from anaerobic oxidations via humic acids towards iron reduction. This information needs to be considered in future studies of electron flow in soils and sediments.
Figures

References
-
- Cummins C S, Johnson J L. The genus Propionibacterium. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 834–849.
-
- Curtis G P, Reinhard M. Reductive dehalogenation of hexachloroethane, carbon tetrachloride, and bromoform by anthrahydroquinone disulfonate and humic acids. Environ Sci Technol. 1994;28:2393–2401. - PubMed
-
- Dannenberg S, Kroder M, Dilling W, Cypionka H. Oxidation of H2, organic compounds and inorganic sulfur compounds coupled to reduction of O2 or nitrate by sulfate-reducing bacteria. Arch Microbiol. 1992;158:93–99.
-
- Dunnivant F M, Schwarzenbach R P, Macalady D L. Reduction of substituted nitrobenzenes in aqueous solutions containing natural organic matter. Environ Sci Technol. 1992;26:2133–2141.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

