Caspase-2 (Nedd-2) processing and death of trophic factor-deprived PC12 cells and sympathetic neurons occur independently of caspase-3 (CPP32)-like activity
- PMID: 9801360
- PMCID: PMC6792876
- DOI: 10.1523/JNEUROSCI.18-22-09204.1998
Caspase-2 (Nedd-2) processing and death of trophic factor-deprived PC12 cells and sympathetic neurons occur independently of caspase-3 (CPP32)-like activity
Abstract
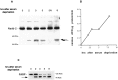
We have previously shown that caspase-2 (Nedd-2) is required for apoptosis induced by withdrawal of trophic support from PC12 cells and sympathetic neurons. Here, we examine the relationship of caspase-2 processing and cell death to induction of caspase-3 (CPP32)-like activity in PC12 cells. Caspase-2 processing, at a site tentatively identified as D333, led to the formation of an N-terminal 37 kDa product. This processing correlated temporally with induction of caspase-3-like activity. Agents previously shown to inhibit caspase-3-like activation, such as bcl-2 and the Cdk inhibitor flavopiridol, also acted upstream of caspase-2 processing. The general caspase inhibitors BAF and zVAD-FMK inhibited N-terminal caspase-2 processing. In contrast, the more selective caspase inhibitor DEVD-FMK inhibited the induction of caspase-3-like activity but did not affect caspase-2 processing or significantly suppress death in PC12 cells or sympathetic neurons. This indicates that caspase-3-like activity is not required for either caspase-2 processing or apoptosis in this paradigm. An antisense oligonucleotide to caspase-2 inhibited cell death but did not affect caspase-3-like activity, indicating that caspase-2 is not upstream of this activity and that activation of caspase-3-like caspases is not sufficient for death. Thus, in our paradigm, caspase-2 processing and caspase-3-like activity are induced independently of each other. Moreover, although death requires caspase-2, caspase-3-like activity is neither necessary nor sufficient for death.
Figures






References
-
- Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein dye-binding. Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
