A lateralized deficit in morphine antinociception after unilateral inactivation of the central amygdala
- PMID: 9801383
- PMCID: PMC6792902
- DOI: 10.1523/JNEUROSCI.18-22-09453.1998
A lateralized deficit in morphine antinociception after unilateral inactivation of the central amygdala
Abstract
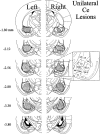
The amygdala is a forebrain region that is receiving increasing attention as a modulator of pain sensation. The amygdala contributes to antinociception elicited by both psychological factors (e.g., fear) and exogenous opioid agonists. Unlike the midbrain periaqueductal gray matter (PAG) or rostral ventromedial medulla, the amygdala is a pain-modulating region that has clear bilateral representation in the brain, making it possible to determine whether pain-modulating effects of this region are lateralized with respect to the peripheral origin of noxious stimulation. Unilateral inactivation of the central nucleus of the amygdala (Ce) plus adjacent portions of the basolateral amygdaloid complex (with either the excitotoxin NMDA or the GABAA agonist muscimol) reduced the ability of morphine to suppress prolonged, formalin-induced pain derived from the hindpaw ipsilateral, but not contralateral, to the inactivated region. This effect was evident regardless of the nociceptive scoring method used (weighted scores or flinch-frequency method) and was not accompanied by a concurrent reduction in morphine-induced hyperlocomotion. Unilateral lesions restricted to the basolateral amygdaloid complex (i.e., not including the Ce) did not reduce the ability of morphine to suppress formalin-induced pain derived from either hindpaw. The results constitute the first report of a lateralized deficit in opioid antinociception after unilateral inactivation of a specific brain area and show the first clear neuroanatomical dissociation between antinociceptive and motor effects of systemically administered morphine in the rat. The amygdala appears to modulate nociceptive signals entering the ipsilateral spinal dorsal horn, probably through monosynaptic connections with ipsilateral portions of the PAG.
Figures















References
-
- Abbott FV, Franklin KBJ, Westbrook RF. The formalin test: scoring properties of the first and second phases of the pain response in rats. Pain. 1995;60:91–102. - PubMed
-
- Abbott FV, Hong Y, Franklin KBJ. The effect of lesions of the dorsolateral funiculus on formalin pain and morphine analgesia: a dose-response analysis. Pain. 1996;65:17–23. - PubMed
-
- Abols IA, Basbaum AI. Afferent connections of the rostral medulla of the cat: a neural substrate for midbrain-medullary interactions in the modulation of pain. J Comp Neurol. 1981;201:285–297. - PubMed
-
- Aggleton JP. The contribution of the amygdala to normal and abnormal emotional states. Trends Neurosci. 1993;16:328–333. - PubMed
-
- Al-Rodhan N, Chipkin R, Yaksh TL. The antinociceptive effects of SCH-32615, a neutral endopeptidase (enkephalinase) inhibitor, microinjected into the periaqueductal, ventral medulla and amygdala. Brain Res. 1990;520:123–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
