Activation of vagal afferents after intravenous injection of interleukin-1beta: role of endogenous prostaglandins
- PMID: 9801384
- PMCID: PMC6792875
- DOI: 10.1523/JNEUROSCI.18-22-09471.1998
Activation of vagal afferents after intravenous injection of interleukin-1beta: role of endogenous prostaglandins
Abstract
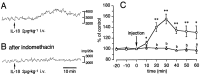
Intravenous administration of interleukin-1 (IL-1) activates central autonomic neuronal circuitries originating in the nucleus of the solitary tract (NTS). The mechanism(s) by which blood-borne IL-1 regulates brain functions, whether by operating across the blood-brain barrier and/or by activating peripheral sensory afferents, remains to be characterized. It has been proposed that vagal afferents originating in the periphery may monitor circulating IL-1 levels, because neurons within the NTS are primary recipients of sensory information from the vagus nerve and also exhibit exquisite sensitivity to blood-borne IL-1. In this study, we present evidence that viscerosensory afferents of the vagus nerve respond to intravenously administered IL-1beta. Specific labeling for mRNAs encoding the type 1 IL-1 receptor and the EP3 subtype of the prostaglandin E2 receptor was detected in situ over neuronal cell bodies in the rat nodose ganglion. Moreover, intravenously applied IL-1 increased the number of sensory neurons in the nodose ganglion that express the cellular activation marker c-Fos, which was matched by an increase in discharge activity of vagal afferents arising from gastric compartments. This response to IL-1 administration was attenuated in animals pretreated with the cyclooxygenase inhibitor indomethacin, suggesting partial mediation by prostaglandins. In conclusion, these results demonstrate that somata and/or fibers of sensory neurons of the vagus nerve express receptors to IL-1 and prostaglandin E2 and that circulating IL-1 stimulates vagal sensory activity via both prostaglandin-dependent and -independent mechanisms.
Figures


References
-
- Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol. 1989;283:248–268. - PubMed
-
- Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. - PubMed
-
- Bergren DR, Gustafson JM, Myers DL. Effect of prostaglandin F2 alpha on pulmonary rapidly-adapting-receptors in the guinea pig. Prostaglandins. 1984;27:391–405. - PubMed
-
- Berthoud HR, Kressel M, Neuhuber WL. Vagal afferent innervation of rat abdominal paraganglia as revealed by anterograde DiI-tracing and confocal microscopy. Acta Anat. 1995;152:127–132. - PubMed
-
- Besedovsky HO, del Rey A. Mechanism of virus-induced stimulation of the hypothalamus–pituitary–adrenal axis. J Steroid Biochem. 1989;34:235–239. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
