Synaptic integration in striate cortical simple cells
- PMID: 9801388
- PMCID: PMC6792880
- DOI: 10.1523/JNEUROSCI.18-22-09517.1998
Synaptic integration in striate cortical simple cells
Abstract
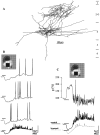
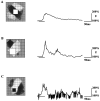
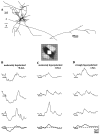
Simple cells in the visual cortex respond to the precise position of oriented contours (Hubel and Wiesel, 1962). This sensitivity reflects the structure of the simple receptive field, which exhibits two sorts of antagonism between on and off inputs. First, simple receptive fields are divided into adjacent on and off subregions; second, within each subregion, stimuli of the reverse contrast evoke responses of the opposite sign: push-pull (Hubel and Wiesel, 1962; Palmer and Davis, 1981; Jones and Palmer, 1987; Ferster, 1988). We have made whole-cell patch recordings from cat area 17 during visual stimulation to examine the generation and integration of excitation (push) and suppression (pull) in the simple receptive field. The temporal structure of the push reflected the pattern of thalamic inputs, as judged by comparing the intracellular cortical responses to extracellular recordings made in the lateral geniculate nucleus. Two mechanisms have been advanced to account for the pull-withdrawal of thalamic drive and active, intracortical inhibition (Hubel and Wiesel, 1962; Heggelund, 1968; Ferster, 1988). Our results suggest that intracortical inhibition is the dominant, and perhaps sole, mechanism of suppression. The inhibitory influences operated within a wide dynamic range. When inhibition was strong, the membrane conductance could be doubled or tripled. Furthermore, if a stimulus confined to one subregion was enlarged so that it extended into the next, the sign of response often changed from depolarizing to hyperpolarizing. In other instances, the inhibition modulated neuronal output subtly, by elevating spike threshold or altering firing rate at a given membrane voltage.
Figures



References
-
- Ahmed B, Anderson JC, Douglas RJ, Martin KM, Nelson JC. Polyneuronal innervation of spiny stellate neurons in cat visual cortex. J Comp Neurol. 1994;341:39–49. - PubMed
-
- Alonso JM, Usrey WM, Reid RC. Precisely correlated firing in cells of the lateral geniculate nucleus. Nature. 1996;383:815–819. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
