Rab11 is required for trans-golgi network-to-plasma membrane transport and a preferential target for GDP dissociation inhibitor
- PMID: 9802909
- PMCID: PMC25617
- DOI: 10.1091/mbc.9.11.3241
Rab11 is required for trans-golgi network-to-plasma membrane transport and a preferential target for GDP dissociation inhibitor
Abstract
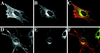
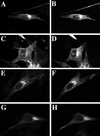
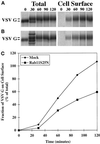
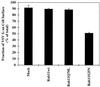
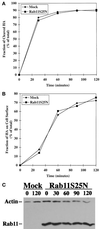
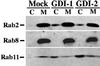
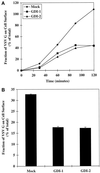
The rab11 GTPase has been localized to both the Golgi and recycling endosomes; however, its Golgi-associated function has remained obscure. In this study, rab11 function in exocytic transport was analyzed by using two independent means to perturb its activity. First, expression of the dominant interfering rab11S25N mutant protein led to a significant inhibition of the cell surface transport of vesicular stomatitis virus (VSV) G protein and caused VSV G protein to accumulate in the Golgi. On the other hand, the expression of wild-type rab11 or the activating rab11Q70L mutant had no adverse effect on VSV G transport. Next, the membrane association of rab11, which is crucial for its function, was perturbed by modest increases in GDP dissociation inhibitor (GDI) levels. This led to selective inhibition of the trans-Golgi network to cell surface delivery, whereas endoplasmic reticulum-to-Golgi and intra-Golgi transport were largely unaffected. The transport inhibition was reversed specifically by coexpression of wild-type rab11 with GDI. Under the same conditions two other exocytic rab proteins, rab2 and rab8, remained membrane bound, and the transport steps regulated by these rab proteins were unaffected. Neither mutant rab11S25N nor GDI overexpression had any impact on the cell surface delivery of influenza hemagglutinin. These data show that functional rab11 is critical for the export of a basolateral marker but not an apical marker from the trans-Golgi network and pinpoint rab11 as a sensitive target for inhibition by excess GDI.
Figures


References
-
- Bachner D, Sedlacek Z, Korn B, Hameister H, Poustka A. Expression patterns of two human genes coding for different rab GDP-dissociation inhibitors (GDIs), extremely conserved proteins involved in cellular transport. Hum Mol Genet. 1995;4:701–708. - PubMed
-
- Brent R, Ptashne M. A eukaryotic transcriptional activator bearing the DNA specificity of a prokaryotic repressor. Cell. 1985;43:729–736. - PubMed
-
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

