Heteroclitic immunization induces tumor immunity
- PMID: 9802967
- PMCID: PMC2212523
- DOI: 10.1084/jem.188.9.1553
Heteroclitic immunization induces tumor immunity
Abstract
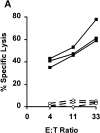
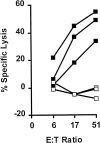
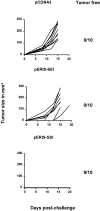
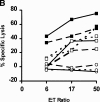
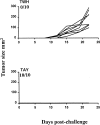
In tumor transplantation models in mice, cytotoxic T lymphocytes (CTLs) are typically the primary effector cells. CTLs recognize major histocompatibility complex (MHC) class I-associated peptides expressed by tumors, leading to tumor rejection. Peptides presented by cancer cells can originate from viral proteins, normal self-proteins regulated during differentiation, or altered proteins derived from genetic alterations. However, many tumor peptides recognized by CTLs are poor immunogens, unable to induce activation and differentiation of effector CTLs. We used MHC binding motifs and the knowledge of class I:peptide:TCR structure to design heteroclitic CTL vaccines that exploit the expression of poorly immunogenic tumor peptides. The in vivo potency of this approach was demonstrated using viral and self-(differentiation) antigens as models. First, a synthetic variant of a viral antigen was expressed as a tumor antigen, and heteroclitic immunization with peptides and DNA was used to protect against tumor challenge and elicit regression of 3-d tumors. Second, a peptide from a relevant self-antigen of the tyrosinase family expressed by melanoma cells was used to design a heteroclitic peptide vaccine that successfully induced tumor protection. These results establish the in vivo applicability of heteroclitic immunization against tumors, including immunity to poorly immunogenic self-proteins.
Figures



References
-
- Bretscher P, Cohn M. A theory of self-nonself discrimination. Science. 1970;169:1042–1049. - PubMed
-
- Jenkins MK, Ashwell JD, Schwartz RH. Allogeneic non-T spleen cells restore the responsiveness of normal T cell clones stimulated with antigen and chemically modified antigen-presenting cells. J Immunol. 1988;140:3324–3330. - PubMed
-
- Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–1356. - PubMed
-
- Nossal GVJ. Negative selection of lymphocytes. Cell. 1994;76:229–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

