Ex vivo staining of metastatic lymph nodes by class I major histocompatibility complex tetramers reveals high numbers of antigen-experienced tumor-specific cytolytic T lymphocytes
- PMID: 9802976
- PMCID: PMC2212507
- DOI: 10.1084/jem.188.9.1641
Ex vivo staining of metastatic lymph nodes by class I major histocompatibility complex tetramers reveals high numbers of antigen-experienced tumor-specific cytolytic T lymphocytes
Abstract
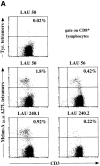
Characterization of cytolytic T lymphocyte (CTL) responses to tumor antigens has been impeded by a lack of direct assays of CTL activity. We have synthesized reagents ("tetramers") that specifically stain CTLs recognizing melanoma antigens. Tetramer staining of tumor-infiltrated lymph nodes ex vivo revealed high frequencies of tumor-specific CTLs which were antigen-experienced by surface phenotype. In vitro culture of lymph node cells with cytokines resulted in very large expansions of tumor-specific CTLs that were dependent on the presence of tumor cells in the lymph nodes. Tetramer-guided sorting by flow cytometer allowed isolation of melanoma-specific CTLs and confirmation of their specificity and their ability to lyse autologous tumor cells. Our results demonstrate the value of these novel reagents for monitoring tumor-specific CTL responses and for generating CTLs for adoptive immunotherapy. These data also indicate that strong CTL responses to melanoma often occur in vivo, and that the reactive CTLs have substantial proliferative and tumoricidal potential.
Figures








References
-
- Robbins PF, Kawakami Y. Human tumor antigens recognized by T cells. Curr Opin Immunol. 1996;8:628–636. - PubMed
-
- Romero P. Cytolytic T lymphocyte responses of cancer patients to tumor-associated antigens. Springer Semin Immunopathol. 1996;18:185–198. - PubMed
-
- Romero P, Cerottini JC, Waanders G. Novel methods to monitor antigen-specific cytotoxic T cell responses in cancer immunotherapy. Mol Med Today. 1998;4:305–312. - PubMed
-
- Altman JD, Moss PAH, Goulder PJR, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. - PubMed
-
- Dunbar PR, Ogg GS, Chen J, Rust N, van der Bruggen P, Cerundolo V. Direct isolation, phenotyping and cloning of low frequency antigen-specific cytotoxic T lymphocytes from peripheral blood. Curr Biol. 1998;8:413–416. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

