Identification of functional human splenic memory B cells by expression of CD148 and CD27
- PMID: 9802981
- PMCID: PMC2212517
- DOI: 10.1084/jem.188.9.1691
Identification of functional human splenic memory B cells by expression of CD148 and CD27
Abstract
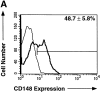
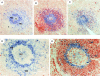
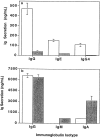
Memory B cells isolated from human tonsils are characterized by an activated cell surface phenotype, localization to mucosal epithelium, expression of somatically mutated immunoglobulin (Ig) variable (V) region genes, and a preferential differentiation into plasma cells in vitro. In spleens of both humans and rodents, a subset of memory B cells is believed to reside in the marginal zone of the white pulp. Similar to tonsil-derived memory B cells, splenic marginal zone B cells can be distinguished from naive follicular B cells by a distinct cell surface phenotype and by the presence of somatic mutations in their Ig V region genes. Although differences exist between human naive and memory B cells, no cell surface molecules have been identified that positively identify all memory B cells. In this study, we have examined the expression of the receptor-type protein tyrosine phosphatase CD148 on human B cells. CD148(+) B cells present in human spleen exhibited characteristics typical of memory B cells. These included an activated phenotype, localization to the marginal zone, the expression of somatically mutated Ig V region genes, and the preferential differentiation into plasma cells. In contrast, CD148(-) B cells appeared to be naive B cells due to localization to the mantle zone, the expression of surface antigens typical of unstimulated B cells, and the expression of unmutated Ig V region genes. Interestingly, CD148(+) B cells also coexpressed CD27, whereas CD148(-) B cells were CD27(-). These results identify CD148 and CD27 as markers which positively identify memory B cells present in human spleen. Thus, assessing expression of these molecules may be a convenient way to monitor the development of memory B cell responses in immunocompromised individuals or in vaccine trials.
Figures











References
-
- MacLennan IC, Liu Y-J, Johnson GD. Maturation and dispersal of B-cell clones during T cell-dependent antibody responses. Immunol Rev. 1992;126:143–161. - PubMed
-
- Banchereau J, Rousset F. Human B lymphocytes: phenotype, proliferation, and differentiation. Adv Immunol. 1992;52:125–262. - PubMed
-
- Gray D. Immunological memory. Annu Rev Immunol. 1993;11:49–77. - PubMed
-
- Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. - PubMed
-
- Liu Y-J, Grouard G, de Bouteiller O, Banchereau J. Follicular dendritic cells and germinal centers. Int Rev Cytol. 1996;166:139–179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

