Rapid secretion of prestored interleukin 8 from Weibel-Palade bodies of microvascular endothelial cells
- PMID: 9802986
- PMCID: PMC2212514
- DOI: 10.1084/jem.188.9.1751
Rapid secretion of prestored interleukin 8 from Weibel-Palade bodies of microvascular endothelial cells
Abstract
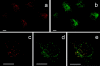
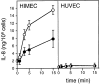
Interleukin (IL)-8, a C-X-C chemokine, activates integrin-mediated adhesion of neutrophils. Presentation of IL-8 on the endothelial cell surface may promote leukocyte extravasation. We found that cultured human microvascular endothelial cells from the intestine (HIMEC) and from nasal polyps (PMEC), but not human umbilical vein endothelial cells (HUVEC), contained IL-8 in intracellular granules that coexpressed von Willebrand factor (vWf ). This observation was corroborated by the immunohistochemical observation of double-positive granules (IL-8(+)vWf+) in vessels of small and large intestine, nasal mucosa, and skin, whereas umbilical cords revealed no endothelial IL-8. After treatment of HIMEC or PMEC with histamine or thrombin, a dramatic increase in supernatant IL-8 concentration was observed within 3 min, whereas no increase in IL-8 was detected in supernatants of identically treated HUVEC cultures. Histamine or thrombin treatment also caused IL-8-containing granules to rapidly disappear from HIMEC. In HUVEC, IL-8-containing granules were inducible by treatment with recombinant human IL-1beta for 24 h; additional histamine treatment doubled IL-8 secretion from HUVEC in the same rapid manner observed for mucosal EC. These data suggested that IL-8 prestored in microvascular endothelial cells may provide a rapid pathway for specific activation of neutrophil adhesion at sites of acute inflammation.
Figures




References
-
- Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. - PubMed
-
- Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. - PubMed
-
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. - PubMed
-
- Bonfanti R, Furie BC, Furie B, Wagner DD. PADGEM (GMP 140) is a component of Weibel-Palade bodies of human endothelial cells. Blood. 1989;73:1109–1112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

