The ovo gene required for cuticle formation and oogenesis in flies is involved in hair formation and spermatogenesis in mice
- PMID: 9808631
- PMCID: PMC317232
- DOI: 10.1101/gad.12.21.3452
The ovo gene required for cuticle formation and oogenesis in flies is involved in hair formation and spermatogenesis in mice
Abstract
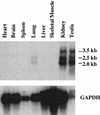
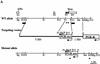
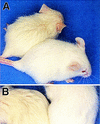
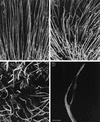
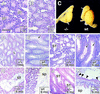
The Drosophila svb/ovo gene gives rise to differentially expressed transcripts encoding a zinc finger protein. svb/ovo has two distinct genetic functions: shavenbaby (svb) is required for proper formation of extracellular projections that are produced by certain epidermal cells in late-stage differentiation; ovo is required for survival and differentiation of female germ cells. We cloned a mouse gene, movo1 encoding a nuclear transcription factor that is highly similar to its fly counterpart in its zinc-finger sequences. In mice, the gene is expressed in skin, where it localizes to the differentiating cells of epidermis and hair follicles, and in testes, where it is present in spermatocytes and spermatids. Using gene targeting, we show that movo1 is required for proper development of both hair and sperm. movo1(-/-) mice are small, produce aberrant hairs, and display hypogenitalism, with a reduced ability to reproduce. These mice also develop abnormalities in kidney, where movo1 is also expressed. Our findings reveal remarkable parallels between mice and flies in epidermal appendage formation and in germ-cell maturation. Furthermore, they uncover a phenotype similar to that of Bardet-Biedl syndrome, a human disorder that maps to the same locus as human ovo1.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
