Involvement of ethylene in potato microtuber dormancy
- PMID: 9808728
- PMCID: PMC34794
- DOI: 10.1104/pp.118.3.843
Involvement of ethylene in potato microtuber dormancy
Abstract
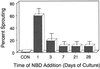
Potato (Solanum tuberosum L.) single-node explants undergoing in vitro tuberization produced detectable amounts of ethylene throughout tuber development, and the resulting microtubers were completely dormant (endodormant) for at least 12 to 15 weeks. The rate of ethylene production by tuberizing explants was highest during the initial 2 weeks of in vitro culture and declined thereafter. Continuous exposure of developing microtubers to the noncompetitive ethylene antagonist AgNO3 via the culture medium resulted in a dose-dependent increase in precocious sprouting. The effect of AgNO3 on the premature loss of microtuber endodormancy was observed after 3 weeks of culture. Similarly, continuous exposure of developing microtubers to the competitive ethylene antagonist 2, 5-norbornadiene (NBD) at concentrations of 2 mL/L (gas phase) or greater also resulted in a dose-dependent increase in premature sprouting. Exogenous ethylene reversed this response and inhibited the precocious sprouting of NBD-treated microtubers. NBD treatment was effective only when it was begun within 7 d of the start of in vitro explant culture. These results indicate that endogenous ethylene is essential for the full expression of potato microtuber endodormancy, and that its involvement may be restricted to the initial period of endodormancy development.
Figures




References
-
- Alam SMM, Murr DP, Kristof L. The effect of ethylene and of inhibitors of protein and nucleic acid syntheses on dormancy break and subsequent sprout growth. Potato Res. 1994;37:25–33.
-
- Burton WG. Studies on the dormancy and sprouting of potatoes. III. The effect upon sprouting of volatile metabolic products other than carbon dioxide. New Phytol. 1952;51:154–162.
-
- Burton WG (1989) The Potato, Ed 3. Longman Scientific & Technical, Essex, UK, pp 470–504
-
- Burton WG, Meigh DF. The production of growth-suppressing volatile substances by stored potato tubers. Potato Res. 1971;14:96–101.
-
- Coleman WK. Dormancy release in potato tubers: a review. Am Potato J. 1987;64:57–68.
LinkOut - more resources
Full Text Sources

