Phosphorylated nitrate reductase and 14-3-3 proteins. Site of interaction, effects of ions, and evidence for an amp-binding site on 14-3-3 proteins
- PMID: 9808749
- PMCID: PMC34777
- DOI: 10.1104/pp.118.3.1041
Phosphorylated nitrate reductase and 14-3-3 proteins. Site of interaction, effects of ions, and evidence for an amp-binding site on 14-3-3 proteins
Abstract
The inactivation of phosphorylated nitrate reductase (NR) by the binding of 14-3-3 proteins is one of a very few unambiguous biological functions for 14-3-3 proteins. We report here that serine and threonine residues at the +6 to +8 positions, relative to the known regulatory binding site involving serine-543, are important in the interaction with GF14omega, a recombinant plant 14-3-3. Also shown is that an increase in ionic strength with KCl or inorganic phosphate, known physical effectors of NR activity, directly disrupts the binding of protein and peptide ligands to 14-3-3 proteins. Increased ionic strength attributable to KCl caused a change in conformation of GF14omega, resulting in reduced surface hydrophobicity, as visualized with a fluorescent probe. Similarly, it is shown that the 5' isomer of AMP was specifically able to disrupt the inactive phosphorylated NR:14-3-3 complex. Using the 5'-AMP fluorescent analog trinitrophenyl-AMP, we show that there is a probable AMP-binding site on GF14omega.
Figures
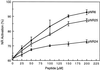
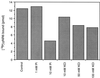
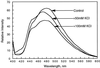
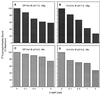
References
-
- Aitken A. 14-3-3 and its possible role in co-ordinating multiple signalling pathways. Trends Cell Biol. 1996;6:341–347. - PubMed
-
- Bachmann M, Huber JL, Athwal GS, Wu K, Ferl RJ, Huber SC. 14-3-3 proteins associated with the regulatory phosphorylation site of spinach leaf nitrate reductase in an isoform-specific manner and reduced dephosphorylation of Ser-543 by endogenous protein phosphatases. FEBS Lett. 1996a;398:26–30. - PubMed
-
- Bachmann M, Huber JL, Liao P-C, Gage DA, Huber SC. The inhibitor protein of phosphorylated nitrate reductase from spinach (Spinacia oleracea) leaves is a 14-3-3 protein. FEBS Lett. 1996b;387:127–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

