Purification, gene cloning, targeted knockout, overexpression, and biochemical characterization of the major pyrazinamidase from Mycobacterium smegmatis
- PMID: 9811635
- PMCID: PMC107651
- DOI: 10.1128/JB.180.22.5809-5814.1998
Purification, gene cloning, targeted knockout, overexpression, and biochemical characterization of the major pyrazinamidase from Mycobacterium smegmatis
Abstract
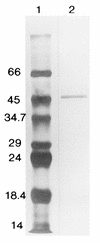
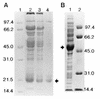
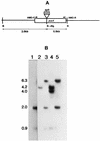
The pyrazinamidase from Mycobacterium smegmatis was purified to homogeneity to yield a product of approximately 50 kDa. The deduced amino-terminal amino acid sequence of this polypeptide was used to design an oligonucleotide probe for screening a DNA library of M. smegmatis. An open reading frame, designated pzaA, which encodes a polypeptide of 49.3 kDa containing motifs conserved in several amidases was identified. Targeted knockout of the pzaA gene by homologous recombination yielded a mutant, pzaA::aph, with a more-than-threefold-reduced level of pyrazinamidase activity, suggesting that this gene encodes the major pyrazinamidase of M. smegmatis. Recombinant forms of the M. smegmatis PzaA and the Mycobacterium tuberculosis pyrazinamidase/nicotinamidase (PncA) were produced in Escherichia coli and were partially purified and compared in terms of their kinetics of nicotinamidase and pyrazinamidase activity. The comparable Km values obtained from this study suggested that the unique specificity of pyrazinamide (PZA) for M. tuberculosis was not based on an unusually high PZA-specific activity of the PncA protein. Overexpression of pzaA conferred PZA susceptibility on M. smegmatis by reducing the MIC of this drug to 150 micrograms/ml.
Figures
References
-
- Boshoff H I, Mizrahi V. Abstracts of the ASM Conference on Tuberculosis: Past, Present and Future 1997. Washington, D.C: American Society for Microbiology; 1997. Characterization of the pyrazinamidase activity of Mycobacterium smegmatis, abstr. B-72; p. 42.
-
- Calbreath D F, Joshi J G. Inhibition of nicotinamidase by nicotinamide adenine dinucleotide. J Biol Chem. 1971;246:4334–4339. - PubMed
-
- Cynamon M H, Klemens S P, Chou T S, Gimi R H, Welch J T. Antimycobacterial activity of a series of pyrazinoic acid esters. J Med Chem. 1992;35:1212–1215. - PubMed
-
- Damato J J, Collins M T, McClatchy J K. Niacin, nitrate and pyrazinamide studies using Middlebrook 7H10 broth. Eur J Clin Microbiol. 1984;3:546–549. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

