Identification of a zinc-specific metalloregulatory protein, Zur, controlling zinc transport operons in Bacillus subtilis
- PMID: 9811636
- PMCID: PMC107652
- DOI: 10.1128/JB.180.22.5815-5821.1998
Identification of a zinc-specific metalloregulatory protein, Zur, controlling zinc transport operons in Bacillus subtilis
Abstract
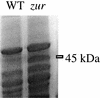
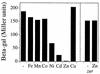
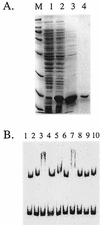
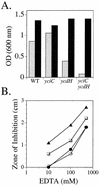
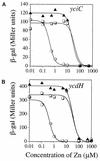
Zinc is an essential nutrient for all cells, but remarkably little is known regarding bacterial zinc transport and its regulation. We have identified three of the key components acting to maintain zinc homeostasis in Bacillus subtilis. Zur is a metalloregulatory protein related to the ferric uptake repressor (Fur) family of regulators and is required for the zinc-specific repression of two operons implicated in zinc uptake, yciC and ycdHIyceA. A zur mutant overexpresses the 45-kDa YciC membrane protein, and purified Zur binds specifically, and in a zinc-responsive manner, to an operator site overlapping the yciC control region. A similar operator precedes the ycdH-containing operon, which encodes an ABC transporter. Two lines of evidence suggest that the ycdH operon encodes a high-affinity zinc transporter whereas YciC may function as part of a lower-affinity pathway. First, a ycdH mutant is impaired in growth in low-zinc medium, and this growth defect is exacerbated by the additional presence of a yciC mutation. Second, mutation of ycdH, but not yciC, alters the regulation of both the yciC and ycdH operons such that much higher levels of exogenous zinc are required for repression. We conclude that Zur is a Fur-like repressor that controls the expression of two zinc homeostasis operons in response to zinc. Thus, Fur-like regulators control zinc homeostasis in addition to their previously characterized roles in regulating iron homeostasis, acid tolerance responses, and oxidative stress functions.
Figures


References
-
- Beard S J, Hashim R, Membrillo-Hernandez J, Hughes M N, Poole R K. Zinc(II) tolerance in Escherichia coli K-12: evidence that the zntA gene (o732) encodes a cation transport ATPase. Mol Microbiol. 1997;25:883–891. - PubMed
-
- Bsat N. Regulation of iron uptake systems in Bacillus subtilis by an iron-specific Fur protein. Ph.D. thesis. Ithaca, N.Y: Cornell University; 1998.
-
- Bsat N, Herbig A, Casillas-Martinez L, Setlow P, Helmann J D. Bacillus subtilis contains multiple Fur homologs: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol Microbiol. 1998;29:189–198. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

