Human immunodeficiency virus induces a dual regulation of Bcl-2, resulting in persistent infection of CD4(+) T- or monocytic cell lines
- PMID: 9811703
- PMCID: PMC110479
- DOI: 10.1128/JVI.72.12.9698-9705.1998
Human immunodeficiency virus induces a dual regulation of Bcl-2, resulting in persistent infection of CD4(+) T- or monocytic cell lines
Abstract
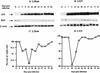
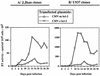
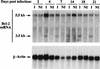
This work aims at characterizing the interplay between human immunodeficiency virus type 1 (HIV-1) and the antiapoptotic cellular protein Bcl-2 responsible for a persistent infection in lymphoblastoid T (J.Jhan) or monocytic (U937) cells. We report that the kinetics of Bcl-2 protein level during the establishment of a chronic infection is biphasic, characterized by a transient decrease followed by restoration to the initial level. The extent and duration of this transient decrease were inversely correlated with the basal level of Bcl-2 as shown by kinetics of Bcl-2 levels in J. Jhan or U937 clones exhibiting different levels of Bcl-2. Using these clones, we also showed that Bcl-2 downregulates HIV-1 replication. Therefore, the cells overexpressing Bcl-2 are characterized by a low viral burden which, in turn, has little effect on the level of this protein. The observed bipasic kinetics is the result of a dual regulation of Bcl-2 induced by HIV-1 infection itself: an upregulation at the transcriptional level of the bcl-2 gene concomitant with a downregulation at the protein level. Convergent data suggest that this downregulation is caused by the oxidative stress induced by the infection itself as shown by the associated modulations of glutathione and thioredoxin levels and by the prevention of these dysregulations by N-acetylcysteine. Altogether, these data indicate that infection first results in a decrease of Bcl-2, permitting an initial boost of replication. Then, as the synthesis at the transcriptional level proceeds, the replication is negatively controlled by Bcl-2 to reach a balance characterized by low virus production and a level of Bcl-2 compatible with cell survival. We suggest that the basal level of Bcl-2, together with infection-inducible transcription factors able to activate bcl-2 gene transcription, is a critical cellular determinant in the tendency toward an acute or a persistent infection.
Figures




References
-
- Alonso S, Minty A, Bourlet Y, Buckingham M. Comparison of three actin-coding sequences in the mouse; evolutionary relationships between the actin genes of warm-blooded vertebrates. J Mol Evol. 1986;23:11–22. - PubMed
-
- Ameisen J C, Capron A. Cell dysfunction and depletion in AIDS: the programmed cell death hypothesis. Immunol Today. 1991;12:102–105. - PubMed
-
- Boudet F, Lecoeur H, Gougeon M L. Apoptosis associated with ex vivo down-regulation of Bcl-2 and up-regulation of Fas in potential cytotoxic CD8+ T lymphocytes during HIV infection. J Immunol. 1996;156:2282–2293. - PubMed
-
- Buhl R, Jaffe H A, Holroyd K J, Wells F B, Mastrangeli A, Saltini C, Cantin A M, Crystal R G. Glutathione deficiency and HIV. Lancet. 1990;335:546. . (Letter.) - PubMed
-
- Cameron P, Pope M, Granelli P A, Steinman R M. Dendritic cells and the replication of HIV-1. J Leukoc Biol. 1996;59:158–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

