Neutralizing monoclonal antibodies block human immunodeficiency virus type 1 infection of dendritic cells and transmission to T cells
- PMID: 9811714
- PMCID: PMC110490
- DOI: 10.1128/JVI.72.12.9788-9794.1998
Neutralizing monoclonal antibodies block human immunodeficiency virus type 1 infection of dendritic cells and transmission to T cells
Abstract
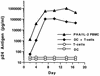
Prevention of the initial infection of mucosal dendritic cells (DC) and interruption of the subsequent transmission of HIV-1 from DC to T cells are likely to be important attributes of an effective human immunodeficiency virus type 1 (HIV-1) vaccine. While anti-HIV-1 neutralizing antibodies have been difficult to elicit by immunization, there are several human monoclonal antibodies (MAbs) that effectively neutralize virus infection of activated T cells. We investigated the ability of three well-characterized neutralizing MAbs (IgG1b12, 2F5, and 2G12) to block HIV-1 infection of human DC. DC were generated from CD14(+) blood cells or obtained from cadaveric human skin. The MAbs prevented viral entry into purified DC and the ensuing productive infection in DC/T-cell cultures. When DC were first pulsed with HIV-1, MAbs blocked the subsequent transmission to unstimulated CD3(+) T cells. Thus, neutralizing antibodies can block HIV-1 infection of DC and the cell-to-cell transmission of virus from infected DC to T cells. These data suggest that neutralizing antibodies could interrupt the initial events associated with mucosal transmission and regional spread of HIV-1.
Figures



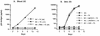
References
-
- Ayehunie S, Garcia-Zepeda E A, Hoxie J A, Horuk R, Kupper T S, Luster A D, Ruprecht R M. Human immunodeficiency virus-1 entry into purified blood dendritic cells through CC and CXC chemokine coreceptors. Blood. 1997;90:1379–1386. - PubMed
-
- Ayehunie S, Groves R W, Bruzzese A-M, Ruprecht R M, Kupper T S, Langhoff E. Acutely infected Langerhans cells are more efficient than T cells in disseminating HIV type 1 to activated T cells following a short cell-cell contact. AIDS Res Hum Retroviruses. 1995;11:877–884. - PubMed
-
- Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Bender A, Sapp M, Schuler G, Steinman R M, Bhardwaj N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Methods. 1996;196:121–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

