Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure
- PMID: 9811715
- PMCID: PMC110491
- DOI: 10.1128/JVI.72.12.9795-9805.1998
Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure
Abstract
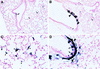
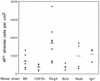
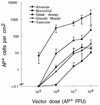
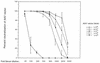
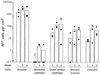
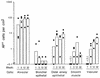
The airway is an important target for gene transfer to treat cystic fibrosis and other diseases that affect the lung. We previously found that marker gene expression did not persist in the bronchial epithelium following adeno-associated virus (AAV) vector administration to the rabbit lung. In an attempt to promote continued expression, we tested repeat vector administration, but no additional transduction was observed, and the block to transduction correlated with the appearance of neutralizing antibodies to the viral capsid. Here we show that mice exhibit a similar response but that treatment with anti-CD40 ligand antibody (MR1) and a soluble CTLA4-immunoglobulin fusion protein (CTLA4Ig) at the time of primary AAV vector exposure allowed successful repeat transduction and prevented production of neutralizing antibodies. We also tested the possibility that an immune response caused the loss of marker-positive cells in the epithelial population in rabbits by evaluating AAV vector expression in immunocompetent and immunodeficient mice. In contrast to results in rabbits, marker protein expression persisted in the lung in both groups of mice. AAV vector transduction occurred in alveolar cells, airway epithelial cells, and smooth muscle cells, and vector expression persisted for at least 8 months. Although data on persistence of AAV vector expression in the human lung are not available, it is likely that repeat transduction will be necessary either due to loss of expression or to the need for repeat administration to deliver effective amounts of AAV vectors. Results presented here indicate that transient immunosuppression will allow such repeat vector treatment of the lung.
Figures

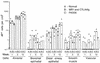
References
-
- Alexander I E, Russell D W, Spence A M, Miller A D. Effects of gamma irradiation on the transduction of dividing and nondividing cells in brain and muscle of rats by adeno-associated virus vectors. Hum Gene Ther. 1996;7:841–850. - PubMed
-
- Ayers M M, Jeffery P K. Proliferation and differentiation in mammalian airway epithelium. Eur Respir J. 1988;1:58–80. - PubMed
-
- Bar D, Tubb J, Ferguson D, Scaria A, Lieber A, Wilson C, Perkins J, Kay M A. Strain related variation in adenovirally mediated transgene expression from mouse hepatocytes in vivo: comparisons between immunocompetent and immunodeficient inbred strains. Gene Ther. 1995;2:151–155. - PubMed
-
- Bierer B E. Mechanisms of action of immunosuppressive agents: cyclosporin A, FK506, and rapamycin. Proc Assoc Am Physicians. 1995;107:28–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

