CCR5- and CXCR4-utilizing strains of human immunodeficiency virus type 1 exhibit differential tropism and pathogenesis in vivo
- PMID: 9811751
- PMCID: PMC110545
- DOI: 10.1128/JVI.72.12.10108-10117.1998
CCR5- and CXCR4-utilizing strains of human immunodeficiency virus type 1 exhibit differential tropism and pathogenesis in vivo
Abstract
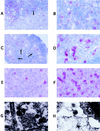
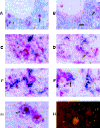
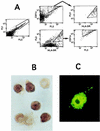
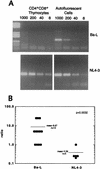
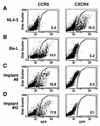
CCR5-utilizing (R5) and CXCR4-utilizing (X4) strains of human immunodeficiency virus type 1 (HIV-1) have been studied intensively in vitro, but the pathologic correlates of such differential tropism in vivo remain incompletely defined. In this study, X4 and R5 strains of HIV-1 were compared for tropism and pathogenesis in SCID-hu Thy/Liv mice, an in vivo model of human thymopoiesis. The X4 strain NL4-3 replicates quickly and extensively in thymocytes in the cortex and medulla, causing significant depletion. In contrast, the R5 strain Ba-L initially infects stromal cells including macrophages in the thymic medulla, without any obvious pathologic consequence. After a period of 3 to 4 weeks, Ba-L infection slowly spreads through the thymocyte populations, occasionally culminating in thymocyte depletion after week 6 of infection. During the entire time of infection, Ba-L did not mutate into variants capable of utilizing CXCR4. Therefore, X4 strains are highly cytopathic after infection of the human thymus. In contrast, infection with R5 strains of HIV-1 can result in a two-phase process in vivo, involving apparently nonpathogenic replication in medullary stromal cells followed by cytopathic replication in thymocytes.
Figures

References
-
- Aldrovandi G M, Feuer G, Gao L, Jamieson B, Kristeva M, Chen I S, Zack J A. The SCID-hu mouse as a model for HIV-1 infection. Nature. 1993;363:732–736. - PubMed
-
- Berkowitz R D, Beckerman K P, Schall T J, McCune J M. CXCR4 and CCR5 expression delineates targets for HIV-1 disruption of T cell differentiation. J Immunol. 1998;161:3702–3710. - PubMed
-
- Berkowitz, R. D., and J. M. McCune. Unpublished data.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

