An endogenous inhibitor of human immunodeficiency virus in human lymphocytes is overcome by the viral Vif protein
- PMID: 9811770
- PMCID: PMC110608
- DOI: 10.1128/JVI.72.12.10251-10255.1998
An endogenous inhibitor of human immunodeficiency virus in human lymphocytes is overcome by the viral Vif protein
Abstract
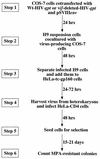
The vif gene of human immunodeficiency virus type 1 (HIV-1) encodes a basic Mr 23,000 protein that is necessary for production of infectious virions by nonpermissive cells (human lymphocytes and macrophages) but not by permissive cells such as HeLa-CD4. It had been proposed that permissive cells may contain an unidentified factor that functions like the viral Vif protein. To test this hypothesis, we produced pseudotyped wild-type and vif-deleted HIV gpt virions (which contain the HIV-1 genome with the bacterial mycophenolic acid resistance gene gpt in place of the viral env gene) in permissive cells, and we used them to generate nonpermissive H9 leukemic T cells that express these proviruses. We then fused these H9 cells with permissive HeLa cells that express the HIV-1 envelope glycoprotein gp120-gp41, and we asked whether the heterokaryons would release infectious HIV gpt virions. The results clearly showed that the vif-deleted virions released by the heterokaryons were noninfectious whereas the wild-type virions were highly infectious. This strongly suggests that nonpermissive cells, the natural targets of HIV-1, contain a potent endogenous inhibitor of HIV-1 replication that is overcome by Vif.
Figures
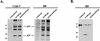


References
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1994.
-
- Blanc D, Patience C, Schulz T F, Weiss R, Spire B. Transcomplementation of VIF− HIV-1 mutants in CEM cells suggests that VIF affects late steps of the viral life cycle. Virology. 1993;193:186–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

