Propagating structure of Alzheimer's beta-amyloid(10-35) is parallel beta-sheet with residues in exact register
- PMID: 9811813
- PMCID: PMC24832
- DOI: 10.1073/pnas.95.23.13407
Propagating structure of Alzheimer's beta-amyloid(10-35) is parallel beta-sheet with residues in exact register
Abstract
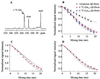
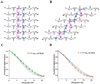
The pathognomonic plaques of Alzheimer's disease are composed primarily of the 39- to 43-aa beta-amyloid (Abeta) peptide. Crosslinking of Abeta peptides by tissue transglutaminase (tTg) indicates that Gln15 of one peptide is proximate to Lys16 of another in aggregated Abeta. Here we report how the fibril structure is resolved by mapping interstrand distances in this core region of the Abeta peptide chain with solid-state NMR. Isotopic substitution provides the source points for measuring distances in aggregated Abeta. Peptides containing a single carbonyl 13C label at Gln15, Lys16, Leu17, or Val18 were synthesized and evaluated by NMR dipolar recoupling methods for the measurement of interpeptide distances to a resolution of 0.2 A. Analysis of these data establish that this central core of Abeta consists of a parallel beta-sheet structure in which identical residues on adjacent chains are aligned directly, i. e., in register. Our data, in conjunction with existing structural data, establish that the Abeta fibril is a hydrogen-bonded, parallel beta-sheet defining the long axis of the Abeta fibril propagation.
Figures


References
-
- Glenner G G, Wong C W. Biochem Biophys Res Commun. 1984;122:1131–1135. - PubMed
-
- Kang J, Lemaire H G, Unterbeck A, Salbaum J M, Masters C L, Grzeschik K H, Multhaup G, Beyreuther K, Muller-Hill B. Nature (London) 1987;325:733–736. - PubMed
-
- Prelli F, Castaño E, Glenner G G, Frangione B. J Neurochem. 1988;51:648–651. - PubMed
-
- Levy E, Carman M D, Fernandez-Madrid I J, Power M D, Lieberburg I, van Duinen S G, Bots G T, Luyendijk W, Frangione B. Science. 1990;248:1124–1126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

