Unblocking of chain-terminated primer by HIV-1 reverse transcriptase through a nucleotide-dependent mechanism
- PMID: 9811824
- PMCID: PMC24843
- DOI: 10.1073/pnas.95.23.13471
Unblocking of chain-terminated primer by HIV-1 reverse transcriptase through a nucleotide-dependent mechanism
Abstract
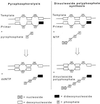
HIV-1 replication is inhibited by the incorporation of chain-terminating nucleotides at the 3' end of the growing DNA chain. Here we show a nucleotide-dependent reaction catalyzed by HIV-1 reverse transcriptase that can efficiently remove the chain-terminating residue, yielding an extendible primer terminus. Radioactively labeled 3'-terminal residue from the primer can be transferred into a product that is resistant to calf intestinal alkaline phosphatase and sensitive to cleavage by snake venom phosphodiesterase. The products formed from different nucleotide substrates have unique electrophoretic migrations and have been identified as dinucleoside tri- or tetraphosphates. The reaction is inhibited by dNTPs that are complementary to the next position on the template (Ki approximately 5 microM), suggesting competition between dinucleoside polyphosphate synthesis and DNA polymerization. Dinucleoside polyphosphate synthesis was inhibited by an HIV-1 specific non-nucleoside inhibitor and was absent in mutant HIV-1 reverse transcriptase deficient in polymerase activity, indicating that this activity requires a functional polymerase active site. We suggest that dinucleoside polyphosphate synthesis occurs by transfer of the 3' nucleotide from the primer to the pyrophosphate moiety in the nucleoside di- or triphosphate substrate through a mechanism analogous to pyrophosphorolysis. Unlike pyrophosphorolysis, however, the reaction is nucleotide-dependent, is resistant to pyrophosphatase, and produces dinucleoside polyphosphates. Because it occurs at physiological concentrations of ribonucleoside triphosphates, this reaction may determine the in vivo activity of many nucleoside antiretroviral drugs.
Figures


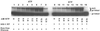

References
-
- Emini E A, Fan H Y. In: Retroviruses. Coffin J M, Hughes S H, Varmus H E, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. pp. 637–706.
-
- Cheng Y-C, Dutschman G E, Bastow K F, Sarngadharan M G, Ting R Y C. J Biol Chem. 1987;262:2187–2189. - PubMed
-
- Huang P, Farquhar D, Plunkett W. J Biol Chem. 1990;265:11914–11918. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

