The lipid phosphatase activity of PTEN is critical for its tumor supressor function
- PMID: 9811831
- PMCID: PMC24850
- DOI: 10.1073/pnas.95.23.13513
The lipid phosphatase activity of PTEN is critical for its tumor supressor function
Abstract
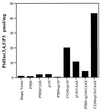
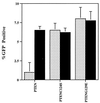
Since their discovery, protein tyrosine phosphatases have been speculated to play a role in tumor suppression because of their ability to antagonize the growth-promoting protein tyrosine kinases. Recently, a tumor suppressor from human chromosome 10q23, called PTEN or MMAC1, has been identified that shares homology with the protein tyrosine phosphatase family. Germ-line mutations in PTEN give rise to several related neoplastic disorders, including Cowden disease. A key step in understanding the function of PTEN as a tumor suppressor is to identify its physiological substrates. Here we report that a missense mutation in PTEN, PTEN-G129E, which is observed in two Cowden disease kindreds, specifically ablates the ability of PTEN to recognize inositol phospholipids as a substrate, suggesting that loss of the lipid phosphatase activity is responsible for the etiology of the disease. Furthermore, expression of wild-type or substrate-trapping forms of PTEN in HEK293 cells altered the levels of the phospholipid products of phosphatidylinositol 3-kinase and ectopic expression of the phosphatase in PTEN-deficient tumor cell lines resulted in the inhibition of protein kinase (PK) B/Akt and regulation of cell survival.
Figures
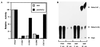

References
-
- Steck P A, Perhouse M A, Jasser S A, Yung W K A, Lin H, Ligon A H, Lauren A L, Baumgard M L, Hattier T, Davis T, et al. Nat Genet. 1997;15:356–362. - PubMed
-
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S, Puc J, Milliaresis C, Rodgers L, McCombie R, et al. Science. 1997;275:1943–1946. - PubMed
-
- Rasheed B K, Stenzel T T, McLendon R E, Parsons R, Friedman A H, Friedman H S, Bigner D D, Bigner S H. Cancer Res. 1997;57:4187–4190. - PubMed
-
- Liaw D, Marsh D J, Li J, Dahia P L M, Wang S I, Zheng Z, Bose S, Call K M, Tsou H C, Peacocke M, et al. Nat Genet. 1997;16:64–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

