A transcriptional activating region with two contrasting modes of protein interaction
- PMID: 9811836
- PMCID: PMC24855
- DOI: 10.1073/pnas.95.23.13543
A transcriptional activating region with two contrasting modes of protein interaction
Abstract
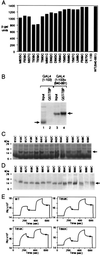
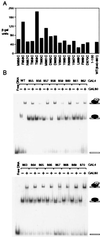
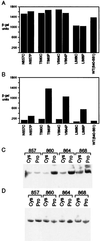
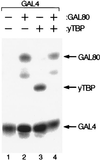
A C-terminal segment of the yeast activator Gal4 manifests two functions: When tethered to DNA, it elicits gene activation, and it binds the inhibitor Gal80. Here we examine the effects on these two functions of cysteine and proline substitutions. We find that, although certain cysteine substitutions diminish interaction with Gal80, those substitutions have little effect on the activating function in vivo and interaction with TATA box-binding protein (TBP) in vitro. Proline substitutions introduced near residues critical for Gal80 binding abolish that interaction but once again have no effect on the activating function. Crosslinking experiments show that a defined position in the activating peptide is in close proximity to TBP and Gal80 in the two separate reactions and show that binding of the inhibitor blocks binding to TBP. Thus, the same stretch of amino acids are involved in two quite different protein-protein interactions: binding to Gal80, which depends on a precise sequence and the formation of a defined secondary structure, or interactions with the transcriptional machinery in vivo, which are not impaired by perturbations of either sequence or structure.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

