TROSY in triple-resonance experiments: new perspectives for sequential NMR assignment of large proteins
- PMID: 9811843
- PMCID: PMC24862
- DOI: 10.1073/pnas.95.23.13585
TROSY in triple-resonance experiments: new perspectives for sequential NMR assignment of large proteins
Abstract
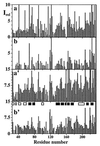
The NMR assignment of 13C, 15N-labeled proteins with the use of triple resonance experiments is limited to molecular weights below approximately 25,000 Daltons, mainly because of low sensitivity due to rapid transverse nuclear spin relaxation during the evolution and recording periods. For experiments that exclusively correlate the amide proton (1HN), the amide nitrogen (15N), and 13C atoms, this size limit has been previously extended by additional labeling with deuterium (2H). The present paper shows that the implementation of transverse relaxation-optimized spectroscopy ([15N,1H]-TROSY) into triple resonance experiments results in several-fold improved sensitivity for 2H/13C/15N-labeled proteins and approximately twofold sensitivity gain for 13C/15N-labeled proteins. Pulse schemes and spectra recorded with deuterated and protonated proteins are presented for the [15N, 1H]-TROSY-HNCA and [15N, 1H]-TROSY-HNCO experiments. A theoretical analysis of the HNCA experiment shows that the primary TROSY effect is on the transverse relaxation of 15N, which is only little affected by deuteration, and predicts sensitivity enhancements that are in close agreement with the experimental data.
Figures




References
-
- Wüthrich K, Wider G, Wagner G, Braun W. J Mol Biol. 1982;155:311–319. - PubMed
-
- Wüthrich K. NMR of Proteins and Nucleic Acids. New York: Wiley; 1986.
-
- Montelione G T, Wagner G. J Am Chem Soc. 1989;111:5474–5475.
-
- Ikura M, Kay L E, Bax A. Biochemistry. 1990;29:4659–4667. - PubMed
-
- Bax A, Grzesiek S. Acc Chem Res. 1993;26:131–138.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

