Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance
- PMID: 9811848
- PMCID: PMC24867
- DOI: 10.1073/pnas.95.23.13612
Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance
Abstract
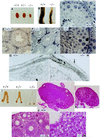
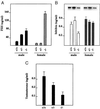
Pituitary gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone stimulate the gonads by regulating germ cell proliferation and differentiation. FSH receptors (FSH-Rs) are localized to testicular Sertoli cells and ovarian granulosa cells and are coupled to activation of the adenylyl cyclase and other signaling pathways. Activation of FSH-Rs is considered essential for folliculogenesis in the female and spermatogenesis in the male. We have generated mice lacking FSH-R by homologous recombination. FSH-R-deficient males are fertile but display small testes and partial spermatogenic failure. Thus, although FSH signaling is not essential for initiating spermatogenesis, it appears to be required for adequate viability and motility of the sperms. FSH-R-deficient females display thin uteri and small ovaries and are sterile because of a block in folliculogenesis before antral follicle formation. Although the expression of marker genes is only moderately altered in FSH-R -/- mice, drastic sex-specific changes are observed in the levels of various hormones. The anterior lobe of the pituitary gland in females is enlarged and reveals a larger number of FSH- and thyroid-stimulating hormone (TSH)-positive cells. The phenotype of FSH-R -/- mice is reminiscent of human hypergonadotropic ovarian dysgenesis and infertility.
Figures




References
-
- Ulloa-Aguirre A, Midgley A R, Beitins I Z, Padmanabhan V. Endocr Rev. 1995;16:765–787. - PubMed
-
- Sprengel R, Braun T, Nikolics K, Segaloff D, Seeburg P H. Mol Endocrinol. 1990;4:525–530. - PubMed
-
- Simoni M, Gromoll J, Nieschlag E. Endocr Rev. 1997;18:739–773. - PubMed
-
- Grasso P, Reichert L E. Endocrinology. 1990;128:949–956. - PubMed
-
- Sairam M R, Jiang L G, Yarney T A, Khan H. Biochem Biophys Res Comm. 1996;226:717–722. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

