Characteristics of virus-specific CD8(+) T cells in the liver during the control and resolution phases of influenza pneumonia
- PMID: 9811883
- PMCID: PMC24906
- DOI: 10.1073/pnas.95.23.13812
Characteristics of virus-specific CD8(+) T cells in the liver during the control and resolution phases of influenza pneumonia
Abstract
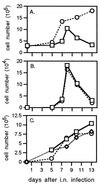
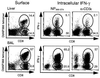
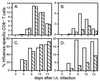
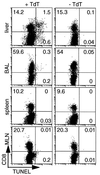
Dissection of the primary and secondary response to an influenza A virus established that the liver contains a substantial population of CD8(+) T cells specific for the immunodominant epitope formed by H-2Db and the influenza virus nucleoprotein peptide fragment NP366-374 (DbNP366). The numbers of CD8(+) DbNP366(+) cells in the liver reflected the magnitude of the inflammatory process in the pneumonic lung, though replication of this influenza virus is limited to the respiratory tract. Analysis of surface phenotypes indicated that the liver CD8(+) DbNP366(+) cells tended to be more "activated" than the set recovered from lymphoid tissue but generally less so than those from the lung. The distinguishing characteristic of the lymphocytes from the liver was that the prevalence of the CD8(+) DbNP366(+) set was always much higher than the percentage of CD8(+) T cells that could be induced to synthesize interferon gamma after short-term, in vitro stimulation with the NP366-374 peptide, whereas these values were generally comparable for virus-specific CD8(+) T cells recovered from other tissue sites. Also, the numbers of apoptotic CD8(+) T cells were higher in the liver. The results overall are consistent with the idea that antigen-specific CD8(+) T cells are destroyed in the liver during the control and resolution phases of this viral infection, though this destruction is not necessarily an immediate process.
Figures


References
-
- Doherty P C, Topham D J, Tripp R A. Immunol Rev. 1996;150:23–44. - PubMed
-
- Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Science. 1996;274:94–96. - PubMed
-
- Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Immunity. 1998;8:177–187. - PubMed
-
- Flynn K J, Belz G T, Altman J D, Ahmed R, Woodland D L, Doherty P C. Immunity. 1998;8:683–691. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

