Impaired liver regeneration in inducible nitric oxide synthasedeficient mice
- PMID: 9811886
- PMCID: PMC24912
- DOI: 10.1073/pnas.95.23.13829
Impaired liver regeneration in inducible nitric oxide synthasedeficient mice
Abstract
The mechanisms that permit adult tissues to regenerate when injured are not well understood. Initiation of liver regeneration requires the injury-related cytokines, tumor necrosis factor (TNF) alpha and interleukin (IL) 6, and involves the activation of cytokine-regulated transcription factors such as NF-kappabeta and STAT3. During regeneration, TNFalpha and IL-6 promote hepatocyte viability, as well as proliferation, because interventions that inhibit either cytokine not only block hepatocyte DNA synthesis, but also increase liver cell death. These observations suggest that the cytokines induce hepatoprotective factors in the regenerating liver. Given evidence that nitric oxide can prevent TNF-mediated activation of the pro-apoptotic protease caspase 3 and protect hepatocytes from cytokine-mediated death, cytokine-inducible nitric oxide synthase (iNOS) may be an important hepatoprotective factor in the regenerating liver. In support of this hypothesis we report that the hepatocyte proliferative response to partial liver resection is severely inhibited in transgenic mice with targeted disruption of the iNOS gene. Instead, partial hepatectomy is followed by increased caspase 3 activity, hepatocyte death, and liver failure, despite preserved induction of TNFalpha, IL-6, NF-kappabeta, and STAT3. These results suggest that during successful tissue regeneration, injury-related cytokines induce factors, such as iNOS and its product, NO, that protect surviving cells from cytokine-mediated death.
Figures
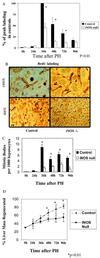



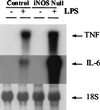
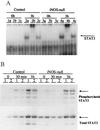

References
-
- Stocum D L. Science. 1997;276:15. - PubMed
-
- Tracey K J, Cerami A. Annu Rev Cell Biol. 1993;9:317–343. - PubMed
-
- Akerman P, Cote P, Yang S Q, McClain C, Nelson S, Bagby G J, Diehl A M. Am J Physiol. 1992;263:G579–G585. - PubMed
-
- Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, Faggioni R, Luini W, van Hinsbergh V, Sozzani S, et al. Immunity. 1997;6:315–325. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

