Dissociation between bone resorption and bone formation in osteopenic transgenic mice
- PMID: 9811887
- PMCID: PMC24916
- DOI: 10.1073/pnas.95.23.13835
Dissociation between bone resorption and bone formation in osteopenic transgenic mice
Abstract
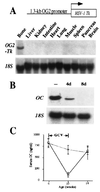
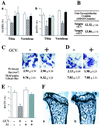
Bone mass is maintained constant in vertebrates through bone remodeling (BR). BR is characterized by osteoclastic resorption of preexisting bone followed by de novo bone formation by osteoblasts. This sequence of events and the fact that bone mass remains constant in physiological situation lead to the assumption that resorption and formation are regulated by each other during BR. Recent evidence shows that cells of the osteoblastic lineage are involved in osteoclast differentiation. However, the existence of a functional link between the two activities, formation and resorption, has never been shown in vivo. To define the role of bone formation in the control of bone resorption, we generated an inducible osteoblast ablation mouse model. These mice developed a reversible osteopenia. Functional analyses showed that in the absence of bone formation, bone resorption continued to occur normally, leading to an osteoporosis of controllable severity, whose appearance could be prevented by an antiresorptive agent. This study establishes that bone formation and/or bone mass do not control the extent of bone resorption in vivo.
Figures


Comment in
-
Bone homeostasis.Proc Natl Acad Sci U S A. 1998 Nov 10;95(23):13361-2. doi: 10.1073/pnas.95.23.13361. Proc Natl Acad Sci U S A. 1998. PMID: 9811806 Free PMC article. No abstract available.
References
-
- Frost H M. Calcif Tissue Res. 1969;3:211–237. - PubMed
-
- Hattner R, Epker B N, Frost H M. Nature (London) 1965;206:489–490. - PubMed
-
- Rodan G A, Martin T J. Calcif Tissue Int. 1981;33:349–351. - PubMed
-
- Rodan G A. In: Osteoporosis. Marcus R, Feldman D, Kelsey J, editors. San Diego: Academic; 1996. pp. 290–301.
-
- Lacey D L, Timms E, Tan H L, Kelley M J, Dunstan C R, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Cell. 1998;93:165–176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

