Catalytically inactive lipoprotein lipase expression in muscle of transgenic mice increases very low density lipoprotein uptake: direct evidence that lipoprotein lipase bridging occurs in vivo
- PMID: 9811888
- PMCID: PMC24920
- DOI: 10.1073/pnas.95.23.13841
Catalytically inactive lipoprotein lipase expression in muscle of transgenic mice increases very low density lipoprotein uptake: direct evidence that lipoprotein lipase bridging occurs in vivo
Abstract
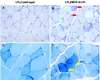
Lipoprotein lipase (LPL) is the central enzyme in plasma triglyceride hydrolysis. In vitro studies have shown that LPL also can enhance lipoprotein uptake into cells via pathways that are independent of catalytic activity but require LPL as a molecular bridge between lipoproteins and proteoglycans or receptors. To investigate whether this bridging function occurs in vivo, two transgenic mouse lines were established expressing a muscle creatine kinase promoter-driven human LPL (hLPL) minigene mutated in the catalytic triad (Asp156 to Asn). Mutated hLPL was expressed only in muscle and led to 3,100 and 3,500 ng/ml homodimeric hLPL protein in post-heparin plasma but no hLPL catalytic activity. Less than 5 ng/ml hLPL was found in preheparin plasma, indicating that proteoglycan binding of mutated LPL was not impaired. Expression of inactive LPL did not rescue LPL knock-out mice from neonatal death. On the wild-type (LPL2) background, inactive LPL decreased very low density lipoprotein (VLDL)-triglycerides. On the heterozygote LPL knock-out background (LPL1) background, plasma triglyceride levels were lowered 22 and 33% in the two transgenic lines. After injection of radiolabeled VLDL, increased muscle uptake was observed for triglyceride-derived fatty acids (LPL2, 1.7x; LPL1, 1.8x), core cholesteryl ether (LPL2, 2.3x; LPL1, 2.7x), and apolipoprotein (LPL1, 1.8x; significantly less than cholesteryl ether). Skeletal muscle from transgenic lines had a mitochondriopathy with glycogen accumulation similar to mice expressing active hLPL in muscle. In conclusion, it appears that inactive LPL can act in vivo to mediate VLDL removal from plasma and uptake into tissues in which it is expressed.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

