Eukaryotic phytochromes: light-regulated serine/threonine protein kinases with histidine kinase ancestry
- PMID: 9811911
- PMCID: PMC24997
- DOI: 10.1073/pnas.95.23.13976
Eukaryotic phytochromes: light-regulated serine/threonine protein kinases with histidine kinase ancestry
Abstract
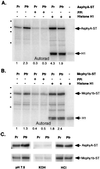
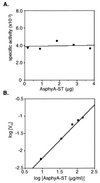
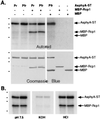
The discovery of cyanobacterial phytochrome histidine kinases, together with the evidence that phytochromes from higher plants display protein kinase activity, bind ATP analogs, and possess C-terminal domains similar to bacterial histidine kinases, has fueled the controversial hypothesis that the eukaryotic phytochrome family of photoreceptors are light-regulated enzymes. Here we demonstrate that purified recombinant phytochromes from a higher plant and a green alga exhibit serine/threonine kinase activity similar to that of phytochrome isolated from dark grown seedlings. Phosphorylation of recombinant oat phytochrome is a light- and chromophore-regulated intramolecular process. Based on comparative protein sequence alignments and biochemical cross-talk experiments with the response regulator substrate of the cyanobacterial phytochrome Cph1, we propose that eukaryotic phytochromes are histidine kinase paralogs with serine/threonine specificity whose enzymatic activity diverged from that of a prokaryotic ancestor after duplication of the transmitter module.
Figures


Comment in
-
Higher-plant phytochrome: "I used to date histidine, but now I prefer serine".Proc Natl Acad Sci U S A. 1998 Nov 10;95(23):13358-60. doi: 10.1073/pnas.95.23.13358. Proc Natl Acad Sci U S A. 1998. PMID: 9811805 Free PMC article. No abstract available.
References
-
- Sage L C. Pigment of the Imagination: A History of Phytochrome Research. San Diego: Academic; 1992.
-
- Smith H. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:289–315.
-
- Pratt L H. Photochem Photobiol. 1995;61:10–21. - PubMed
-
- Furuya M, Schafer E. Trends Plant Sci. 1996;1:301–307.
-
- Quail P H. Plant Cell Environ. 1997;20:657–665.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

