Cell cycle-related changes in the conducting properties of r-eag K+ channels
- PMID: 9813096
- PMCID: PMC2148139
- DOI: 10.1083/jcb.143.3.767
Cell cycle-related changes in the conducting properties of r-eag K+ channels
Abstract
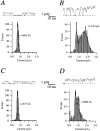
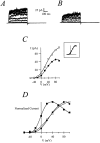
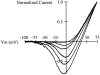
Release from arrest in G2 phase of the cell cycle causes profound changes in rat ether-à-go-go (r-eag) K+ channels heterologously expressed in Xenopus oocytes. The most evident consequence of the onset of maturation is the appearance of rectification in the r-eag current. The trigger for these changes is located downstream of the activation of mitosis-promoting factor (MPF). We demonstrate here that the rectification is due to a voltage-dependent block by intracellular Na+ ions. Manipulation of the intracellular Na+ concentration indicates that the site of Na+ block is located approximately 45% into the electrical distance of the pore and is only present in oocytes undergoing maturation. Since the currents through excised patches from immature oocytes exhibited a fast rundown, we studied CHO-K1 cells permanently transfected with r-eag. These cells displayed currents with a variable degree of block by Na+ and variable permeability to Cs+. Partial synchronization of the cultures in G0/G1 or M phases of the cell cycle greatly reduced the variability. The combined data obtained from mammalian cells and oocytes strongly suggest that the permeability properties of r-eag K+ channels are modulated during cell cycle-related processes.
Figures






References
-
- Baukrowitz T, Yellen G. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 1995;15:951–960. - PubMed
-
- Borin M, Siffert W. Stimulation by thrombin increases the cytosolic free Na+ concentration in human platelets. Studies with the novel fluorescent cytosolic Na+ indicator Na+-binding benzofuran isophthalate. J Biol Chem. 1990;265:19543–19550. - PubMed
-
- Brüggemann A, Pardo LA, Stühmer W, Pongs O. Ether-à-go-go encodes a voltage-gated channel permeable to K+ and Ca 2+and modulated by cAMP. Nature. 1993;365:445–448. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

