TGF-beta plays a key role in morphogenesis of the pancreatic islets of Langerhans by controlling the activity of the matrix metalloproteinase MMP-2
- PMID: 9813100
- PMCID: PMC2148155
- DOI: 10.1083/jcb.143.3.827
TGF-beta plays a key role in morphogenesis of the pancreatic islets of Langerhans by controlling the activity of the matrix metalloproteinase MMP-2
Abstract
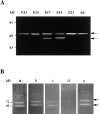
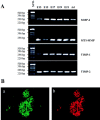
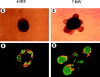
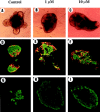
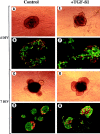
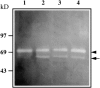
Islets of Langerhans are microorgans scattered throughout the pancreas, and are responsible for synthesizing and secreting pancreatic hormones. While progress has recently been made concerning cell differentiation of the islets of Langerhans, the mechanism controlling islet morphogenesis is not known. It is thought that these islets are formed by mature cell association, first differentiating in the primitive pancreatic epithelium, then migrating in the extracellular matrix, and finally associating into islets of Langerhans. This mechanism suggests that the extracellular matrix has to be degraded for proper islet morphogenesis. We demonstrated in the present study that during rat pancreatic development, matrix metalloproteinase 2 (MMP-2) is activated in vivo between E17 and E19 when islet morphogenesis occurs. We next demonstrated that when E12.5 pancreatic epithelia develop in vitro, MMP-2 is activated in an in vitro model that recapitulates endocrine pancreas development (Miralles, F., P. Czernichow, and R. Scharfmann. 1998. Development. 125: 1017-1024). On the other hand, islet morphogenesis was impaired when MMP-2 activity was inhibited. We next demonstrated that exogenous TGF-beta1 positively controls both islet morphogenesis and MMP-2 activity. Finally, we demonstrated that both islet morphogenesis and MMP-2 activation were abolished in the presence of a pan-specific TGF-beta neutralizing antibody. Taken together, these observations demonstrate that in vitro, TGF-beta is a key activator of pancreatic MMP-2, and that MMP-2 activity is necessary for islet morphogenesis.
Figures


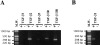
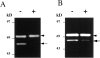

References
-
- Ahlgren U, Pfaff S, Jessel T, Edlund T, Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385:257–260. - PubMed
-
- Atouf F, Czernichow P, Scharfmann R. Expression of neuronal traits in pancreatic beta cells: implication of NRSF/REST, a neuron-restrictive silencer. J Biol Chem. 1997;272:1929–1934. - PubMed
-
- Bottinger E, Jakubczak J, Roberts I, Mumy M, Hemmati P, Bagnall K, Merlino G, Wakefield L. Expression of a dominant-negative mutant TGF-β type II receptor in transgenic mice reveals essential roles for TGF-β in regulation of growth and differentiation in the exocrine pancreas. EMBO (Eur Mol Biol Organ) J. 1997;16:2621–2633. - PMC - PubMed
-
- Chin J, Werb Z. Matrix metalloproteinases regulate morphogenesis, migration and remodeling of epithelium, tongue skeletal muscle and cartilage in the mandibular arch. Development. 1997;124:1519–1530. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

