Impaired integrin-mediated adhesion and signaling in fibroblasts expressing a dominant-negative mutant PTP1B
- PMID: 9813103
- PMCID: PMC2148148
- DOI: 10.1083/jcb.143.3.861
Impaired integrin-mediated adhesion and signaling in fibroblasts expressing a dominant-negative mutant PTP1B
Erratum in
- J Cell Biol 1998 Dec 14;143(6):1761
Abstract
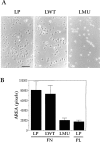
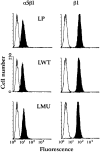
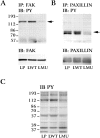
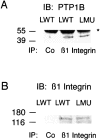
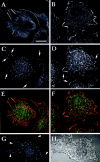
To investigate the role of nonreceptor protein tyrosine phosphatase 1B (PTP1B) in beta1-integrin- mediated adhesion and signaling, we transfected mouse L cells with normal and catalytically inactive forms of the phosphatase. Parental cells and cells expressing the wild-type or mutant PTP1B were assayed for (a) adhesion, (b) spreading, (c) presence of focal adhesions and stress fibers, and (d) tyrosine phosphorylation. Parental cells and cells expressing wild-type PTP1B show similar morphology, are able to attach and spread on fibronectin, and form focal adhesions and stress fibers. In contrast, cells expressing the inactive PTP1B have a spindle-shaped morphology, reduced adhesion and spreading on fibronectin, and almost a complete absence of focal adhesions and stress fibers. Attachment to fibronectin induces tyrosine phosphorylation of focal adhesion kinase (FAK) and paxillin in parental cells and cells transfected with the wild-type PTP1B, while in cells transfected with the mutant PTP1B, such induction is not observed. Additionally, in cells expressing the mutant PTP1B, tyrosine phosphorylation of Src is enhanced and activity is reduced. Lysophosphatidic acid temporarily reverses the effects of the mutant PTP1B, suggesting the existence of a signaling pathway triggering focal adhesion assembly that bypasses the need for active PTP1B. PTP1B coimmunoprecipitates with beta1-integrin from nonionic detergent extracts and colocalizes with vinculin and the ends of actin stress fibers in focal adhesions. Our data suggest that PTP1B is a critical regulatory component of integrin signaling pathways, which is essential for adhesion, spreading, and formation of focal adhesions.
Figures






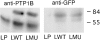



References
-
- Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–523. - PubMed
-
- Bandyopadhyay D, Kusari A, Kenner KA, Liu F, Chernoff J, Gustafson TA, Kusari J. Protein-tyrosine phosphatase 1B complexes with the insulin receptor in vivo and is tyrosine-phosphorylated in the presence of insulin. J Biol Chem. 1997;272:1639–1645. - PubMed
-
- Barry ST, Critchley DR. The rho-dependent assembly of focal adhesions in Swiss 3T3 cells is associated with increased tyrosine phosphorylation and the recruitment of both pp125FAK and protein kinase C-γ to focal adhesions. J Cell Sci. 1994;107:2033–2045. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

