The JNK pathway regulates the In vivo deletion of immature CD4(+)CD8(+) thymocytes
- PMID: 9815259
- PMCID: PMC2212412
- DOI: 10.1084/jem.188.10.1817
The JNK pathway regulates the In vivo deletion of immature CD4(+)CD8(+) thymocytes
Abstract
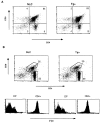
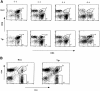
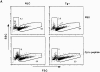
The extracellular signal-regulated kinase (ERK), the c-Jun NH2-terminal kinase (JNK), and p38 MAP kinase pathways are triggered upon ligation of the antigen-specific T cell receptor (TCR). During the development of T cells in the thymus, the ERK pathway is required for differentiation of CD4(-)CD8(-) into CD4(+)CD8(+) double positive (DP) thymocytes, positive selection of DP cells, and their maturation into CD4(+) cells. However, the ERK pathway is not required for negative selection. Here, we show that JNK is activated in DP thymocytes in vivo in response to signals that initiate negative selection. The activation of JNK in these cells appears to be mediated by the MAP kinase kinase MKK7 since high levels of MKK7 and low levels of Sek-1/MKK4 gene expression were detected in thymocytes. Using dominant negative JNK transgenic mice, we show that inhibition of the JNK pathway reduces the in vivo deletion of DP thymocytes. In addition, the increased resistance of DP thymocytes to cell death in these mice produces an accelerated reconstitution of normal thymic populations upon in vivo DP elimination. Together, these data indicate that the JNK pathway contributes to the deletion of DP thymocytes by apoptosis in response to TCR-derived and other thymic environment- mediated signals.
Figures




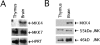










References
-
- Fink PJ, Bevan MJ. Positive selection of thymocytes. Adv Immunol. 1995;59:99–133. - PubMed
-
- Kisielow P, von Boehmer H. Development and selection of T cells: facts and puzzles. Adv Immunol. 1995;58:87–209. - PubMed
-
- Nossal GJV. Negative selection of lymphocytes. Cell. 1994;76:229–239. - PubMed
-
- Whitmarsh AJ, Davis RJ. Transcription factor AP-1: regulation by mitogen activated protein kinases signal transduction pathways. J Mol Med. 1996;17:2360–2371. - PubMed
-
- Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK): from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

