Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis
- PMID: 9815271
- PMCID: PMC2212417
- DOI: 10.1084/jem.188.10.1941
Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis
Abstract
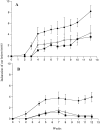
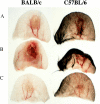
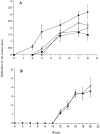
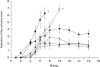
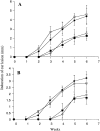
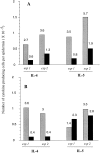
We have developed a model of cutaneous leishmaniasis due to Leishmania major that seeks to mimic the natural conditions of infection. 1,000 metacyclic promastigotes were coinoculated with a salivary gland sonicate (SGS) obtained from a natural vector, Phlebotomus papatasii, into the ear dermis of naive mice or of mice preexposed to SGS. The studies reveal a dramatic exacerbating effect of SGS on lesion development in the dermal site, and a complete abrogation of this effect in mice preexposed to salivary components. In both BALB/c and C57Bl/6 (B/6) mice, the dermal lesions appeared earlier, were more destructive, and contained greater numbers of parasites after infection in the presence of SGS. Furthermore, coinoculation of SGS converted B/6 mice into a nonhealing phenotype. No effect of SGS was seen in either IL-4- deficient or in SCID mice. Disease exacerbation in both BALB/c and B/6 mice was associated with an early (6 h) increase in the frequency of epidermal cells producing type 2 cytokines. SGS did not elicit type 2 cytokines in the epidermis of mice previously injected with SGS. These mice made antisaliva antibodies that were able to neutralize the ability of SGS to enhance infection and to elicit IL-4 and IL-5 responses in the epidermis. These results are the first to suggest that for individuals at risk of vector-borne infections, history of exposure to vector saliva might influence the outcome of exposure to transmitted parasites.
Figures




References
-
- Warburg A, Schlein Y. The effect of post-bloodmeal nutrition of Phlebotomus papatasii on the transmission of Leishmania major. . Am J Trop Med Hyg. 1986;35:926–930. - PubMed
-
- Ribeiro JMC. Role of saliva in blood-feeding by arthropods. Annu Rev Entomol. 1987;32:463–478. - PubMed
-
- Hall LR, Titus RG. Sand fly saliva selectively modulates macrophage functions that inhibit killing of Leishmania majorand nitric oxide production. J Immunol. 1995;155:3501–3506. - PubMed
-
- Titus RG. Salivary gland lysate from the Sand fly Lutzomia longipalpissuppresses the immune response of mice to sheep red blood cells in vivo and concanavalin A in vitro. Exp Parasitol. 1998;89:133–136. - PubMed
-
- Wikel, S.K. 1996. Immunology of the skin. In The Immunology of Host-Ectoparasitic Arthropod Relationships. S.K. Wikel, editor. CAB International, Wallingford, U.K. 1–29.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

