Type III InsP3 receptor channel stays open in the presence of increased calcium
- PMID: 9817204
- PMCID: PMC2825878
- DOI: 10.1038/23954
Type III InsP3 receptor channel stays open in the presence of increased calcium
Abstract
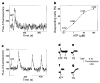
The inositol 1,4,5-trisphosphate receptor (InsP3R) is the main calcium(Ca2+) release channel in most tissues. Three isoforms have been identified, but only types I and II InsP3R have been characterized. Here we examine the functional properties of the type III InsP3R because this receptor is restricted to the trigger zone from which Ca2+ waves originate and it has distinctive InsP3-binding properties. We find that type III InsP3R forms Ca2+ channels with single-channel currents that are similar to those of type I InsP3R; however, the open probability of type III InsP3R isoform increases monotonically with increased cytoplasmic Ca2+ concentration, whereas the type I isoform has a bell-shaped dependence on cytoplasmic Ca2+. The properties of type III InsP3R provide positive feedback as Ca2+ is released; the lack of negative feedback allows complete Ca2+ release from intracellular stores. Thus, activation of type III InsP3R in cells that express only this isoform results in a single transient, but global, increase in the concentration of cytosolic Ca2+. The bell-shaped Ca2+-dependence curve of type I InsP3R is ideal for supporting Ca2+ oscillations, whereas the properties of type III InsP3R are better suited to signal initiation.
Figures


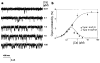
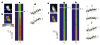
References
-
- Furuichi T, et al. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989;342:32–38. - PubMed
-
- Mignery G, Sudhof TC, Takei K, De Camilli P. Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature. 1989;342:192–195. - PubMed
-
- Blondel O, Takeda J, Janssen H, Seino S, Bell GI. Sequence and functional characterization of a third inositol trisphospate receptor subtype, IP3R-3, expressed in pancreatic islets, gastrointestinal tract, and other tissues. J Biol Chem. 1993;268:11356–11363. - PubMed
-
- Maranto AR. Primary structure, ligand binding, and localization of the human type 3 inositol 1,4,5-trisphosphate receptor expressed in intestinal epithelium. J Biol Chem. 1994;269:1222–1230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

