Armored RNA technology for production of ribonuclease-resistant viral RNA controls and standards
- PMID: 9817878
- PMCID: PMC105245
- DOI: 10.1128/JCM.36.12.3590-3594.1998
Armored RNA technology for production of ribonuclease-resistant viral RNA controls and standards
Abstract
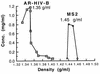
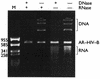
The widespread use of sensitive assays for the detection of viral and cellular RNA sequences has created a need for stable, well-characterized controls and standards. We describe the development of a versatile, novel system for creating RNase-resistant RNA. "Armored RNA" is a complex of MS2 bacteriophage coat protein and RNA produced in Escherichia coli by the induction of an expression plasmid that encodes the coat protein and an RNA standard sequence. The RNA sequences are completely protected from RNase digestion within the bacteriophage-like complexes. As a prototype, a 172-base consensus sequence from a portion of the human immunodeficiency virus type 1 (HIV-1) gag gene was synthesized and cloned into the packaging vector used to produce the bacteriophage-like particles. After production and purification, the resulting HIV-1 Armored RNA particles were shown to be resistant to degradation in human plasma and produced reproducible results in the Amplicor HIV-1 Monitor assay for 180 days when stored at -20 degreesC or for 60 days at 4 degreesC. Additionally, Armored RNA preparations are homogeneous and noninfectious.
Figures
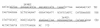



References
-
- Argetsinger J E, Gussin G. Intact ribonucleic acid from defective particles of bacteriophage R17. J Mol Biol. 1966;21:421–434. - PubMed
-
- DuBois D B, WalkerPeach C, Pasloske B L, Winkler M. Universal ribonuclease resistant RNA standards (Armored RNA) for RT-PCR and bDNA-based hepatitis virus RNA assays. Clin Chem. 1997;43:2218. . (Abstract 28.) - PubMed
-
- DuBois, D. B., M. M. Winkler, and B. L. Pasloske. October 1997. U.S. patent 5,677,124.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

