The putative nucleic acid helicase Sen1p is required for formation and stability of termini and for maximal rates of synthesis and levels of accumulation of small nucleolar RNAs in Saccharomyces cerevisiae
- PMID: 9819377
- PMCID: PMC109272
- DOI: 10.1128/MCB.18.12.6885
The putative nucleic acid helicase Sen1p is required for formation and stability of termini and for maximal rates of synthesis and levels of accumulation of small nucleolar RNAs in Saccharomyces cerevisiae
Erratum in
- Mol Cell Biol 1999 Jul;19(7):5235
Abstract
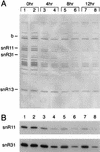
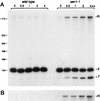
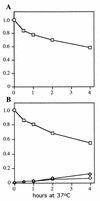
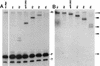
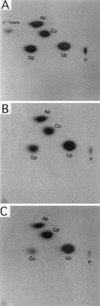
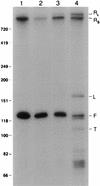
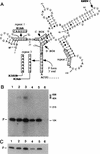
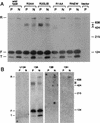
Sen1p from Saccharomyces cerevisiae is a nucleic acid helicase related to DEAD box RNA helicases and type I DNA helicases. The temperature-sensitive sen1-1 mutation located in the helicase motif alters the accumulation of pre-tRNAs, pre-rRNAs, and some small nuclear RNAs. In this report, we show that cells carrying sen1-1 exhibit altered accumulation of several small nucleolar RNAs (snoRNAs) immediately upon temperature shift. Using Northern blotting, RNase H cleavage, primer extension, and base compositional analysis, we detected three forms of the snoRNA snR13 in wild-type cells: an abundant TMG-capped 124-nucleotide (nt) mature form (snR13F) and two less abundant RNAs, including a heterogeneous population of approximately 1,400-nt 3'-extended forms (snR13R) and a 108-nt 5'-truncated form (snR13T) that is missing 16 nt at the 5' end. A subpopulation of snR13R contains the same 5' truncation. Newly synthesized snR13R RNA accumulates with time at the expense of snR13F following temperature shift of sen1-1 cells, suggesting a possible precursor-product relationship. snR13R and snR13T both increase in abundance at the restrictive temperature, indicating that Sen1p stabilizes the 5' end and promotes maturation of the 3' end. snR13F contains canonical C and D boxes common to many snoRNAs. The 5' end of snR13T and the 3' end of snR13F reside within C2U4 sequences that immediately flank the C and D boxes. A mutation in the 5' C2U4 repeat causes underaccumulation of snR13F, whereas mutations in the 3' C2U4 repeat cause the accumulation of two novel RNAs that migrate in the 500-nt range. At the restrictive temperature, double mutants carrying sen1-1 and mutations in the 3' C2U4 repeat show reduced accumulation of the novel RNAs and increased accumulation of snR13R RNA, indicating that Sen1p and the 3' C2U4 sequence act in a common pathway to facilitate 3' end formation. Based on these findings, we propose that Sen1p and the C2U4 repeats that flank the C and D boxes promote maturation of the 3' terminus and stability of the 5' terminus and are required for maximal rates of synthesis and levels of accumulation of mature snR13F.
Figures


Similar articles
-
Sen1p performs two genetically separable functions in transcription and processing of U5 small nuclear RNA in Saccharomyces cerevisiae.Genetics. 2010 Jan;184(1):107-18. doi: 10.1534/genetics.109.110031. Epub 2009 Nov 2. Genetics. 2010. PMID: 19884310 Free PMC article.
-
The yeast SEN1 gene is required for the processing of diverse RNA classes.Nucleic Acids Res. 1997 Dec 1;25(23):4778-85. doi: 10.1093/nar/25.23.4778. Nucleic Acids Res. 1997. PMID: 9365256 Free PMC article.
-
Multiple protein/protein and protein/RNA interactions suggest roles for yeast DNA/RNA helicase Sen1p in transcription, transcription-coupled DNA repair and RNA processing.Nucleic Acids Res. 2004 Apr 30;32(8):2441-52. doi: 10.1093/nar/gkh561. Print 2004. Nucleic Acids Res. 2004. PMID: 15121901 Free PMC article.
-
Yeast exosome mutants accumulate 3'-extended polyadenylated forms of U4 small nuclear RNA and small nucleolar RNAs.Mol Cell Biol. 2000 Jan;20(2):441-52. doi: 10.1128/MCB.20.2.441-452.2000. Mol Cell Biol. 2000. PMID: 10611222 Free PMC article.
-
Inactivation of the yeast Sen1 protein affects the localization of nucleolar proteins.Mol Gen Genet. 1995 Dec 20;249(6):571-84. doi: 10.1007/BF00418026. Mol Gen Genet. 1995. PMID: 8544822
Cited by
-
Protein trans-acting factors involved in ribosome biogenesis in Saccharomyces cerevisiae.Mol Cell Biol. 1999 Dec;19(12):7897-912. doi: 10.1128/MCB.19.12.7897. Mol Cell Biol. 1999. PMID: 10567516 Free PMC article. Review. No abstract available.
-
SUMOylated Senataxin functions in genome stability, RNA degradation, and stress granule disassembly, and is linked with inherited ataxia and motor neuron disease.Mol Genet Genomic Med. 2021 Dec;9(12):e1745. doi: 10.1002/mgg3.1745. Epub 2021 Jul 14. Mol Genet Genomic Med. 2021. PMID: 34263556 Free PMC article. Review.
-
Dhr1p, a putative DEAH-box RNA helicase, is associated with the box C+D snoRNP U3.Mol Cell Biol. 2000 Oct;20(19):7238-46. doi: 10.1128/MCB.20.19.7238-7246.2000. Mol Cell Biol. 2000. PMID: 10982841 Free PMC article.
-
Sen1p performs two genetically separable functions in transcription and processing of U5 small nuclear RNA in Saccharomyces cerevisiae.Genetics. 2010 Jan;184(1):107-18. doi: 10.1534/genetics.109.110031. Epub 2009 Nov 2. Genetics. 2010. PMID: 19884310 Free PMC article.
-
In cis autosomal dominant mutation of Senataxin associated with tremor/ataxia syndrome.Neurogenetics. 2007 Jan;8(1):45-9. doi: 10.1007/s10048-006-0067-8. Epub 2006 Nov 10. Neurogenetics. 2007. PMID: 17096168
References
-
- Balakin A G, Smith L, Fournier M J. The RNA world of the nucleolus: two major families of small RNAs defined by different box elements with related functions. Cell. 1996;86:823–834. - PubMed
-
- Brownlee G G. Determination of Sequences in RNA. New York, N.Y: American Elsevier Publishing; 1972.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
