p21(WAF1/CIP1) is upregulated by the geranylgeranyltransferase I inhibitor GGTI-298 through a transforming growth factor beta- and Sp1-responsive element: involvement of the small GTPase rhoA
- PMID: 9819384
- PMCID: PMC109279
- DOI: 10.1128/MCB.18.12.6962
p21(WAF1/CIP1) is upregulated by the geranylgeranyltransferase I inhibitor GGTI-298 through a transforming growth factor beta- and Sp1-responsive element: involvement of the small GTPase rhoA
Abstract
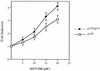
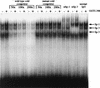
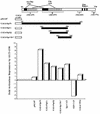
We have recently reported that the geranylgeranyltransferase I inhibitor GGTI-298 arrests human tumor cells at the G1 phase of the cell cycle and increases the protein and RNA levels of the cyclin-dependent kinase inhibitor p21(WAF1/CIP1). Here, we show that GGTI-298 acts at the transcriptional level to induce p21(WAF1/CIP1) in a human pancreatic carcinoma cell line, Panc-1. This upregulation of p21(WAF1/CIP1) promoter was selective, since GGTI-298 inhibited serum responsive element- and E2F-mediated transcription. A functional analysis of the p21(WAF1/CIP1) promoter showed that a GC-rich region located between positions -83 and -74, which contains a transforming growth factor beta-responsive element and one Sp1-binding site, is sufficient for the upregulation of p21(WAF1/CIP1) promoter by GGTI-298. Electrophoretic mobility shift assays showed a small increase in the amount of DNA-bound Sp1-Sp3 complexes. Furthermore, the analysis of Sp1 transcriptional activity in GGTI-298-treated cells by using GAL4-Sp1 chimera or Sp1-chloramphenicol acetyltransferase reporter revealed a significant increase in Sp1-mediated transcription. Moreover, GGTI-298 treatment also resulted in increased Sp1 and Sp3 phosphorylation. These results suggest that GGTI-298-mediated upregulation of p21(WAF1/CIP1) involves both an increase in the amount of DNA-bound Sp1-Sp3 and enhancement of Sp1 transcriptional activity. To identify the geranylgeranylated protein(s) involved in p21(WAF1/CIP1) transcriptional activation, we analyzed the effects of the small GTPases Rac1 and RhoA on p21(WAF1/CIP1) promoter activity. The dominant negative mutant of RhoA, but not Rac1, was able to activate p21(WAF1/CIP1). In contrast, constitutively active RhoA repressed p21(WAF1/CIP1). Accordingly, the ADP-ribosyl transferase C3, which specifically inhibits Rho proteins, enhanced the activity of p21(WAF1/CIP1). Taken together, these results suggest that one mechanism by which GGTI-298 upregulates p21(WAF1/CIP1) transcription is by preventing the small GTPase RhoA from repressing p21(WAF1/CIP1) induction.
Figures



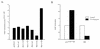
References
-
- Adnane J, Shao Z, Robbins P D. The retinoblastoma susceptibility gene product represses transcription when directly bound to the promoter. J Biol Chem. 1995;270:8837–8843. - PubMed
-
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. - PubMed
-
- Bos J L. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. - PubMed
-
- Cornwell M M, Smith D E. SP1 activates the MDR1 promoter through one of two distinct G-rich regions that modulate promoter activity. J Biol Chem. 1993;268:19505–19511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
