C/EBPalpha is critical for the neonatal acute-phase response to inflammation
- PMID: 9819413
- PMCID: PMC109308
- DOI: 10.1128/MCB.18.12.7269
C/EBPalpha is critical for the neonatal acute-phase response to inflammation
Abstract
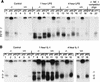
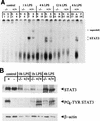
Members of the C/EBP (CCAAT/enhancer binding protein) family of transcription factors play important roles in mediating the acute-phase response (APR), an inflammatory process resulting from infection and/or tissue damage. Among the C/EBP family of proteins, C/EBPbeta and -delta were thought to be the primary mediators of the APR. The function of C/EBPalpha in the APR has not been fully characterized to date. Here, we investigate the role of C/EBPalpha in the APR by using neonatal mice that lack C/EBPalpha expression. Northern blot analysis of acute-phase protein gene expression in neonatal mice treated with purified bacterial lipopolysaccharide or recombinant interleukin 1beta as an inflammation stimulus showed a strong APR in wild-type mice, but a response in C/EBPalpha null animals was completely lacking. The C/EBPalpha knockout and wild-type mice demonstrated elevations in C/EBPbeta and -delta mRNA expression and DNA binding as well as increased DNA binding of NF-kappaB, all of which are known to be important in the APR. Null mice, however, failed to activate STAT3 binding in response to lipopolysaccharide. Our results provide the first evidence that C/EBPalpha is absolutely required for the APR in neonatal mice, is involved in STAT3 regulation, and cannot be compensated for by other C/EBP family members.
Figures



References
-
- Akira S, Kishimoto T. IL-6 and NF-IL6 in acute-phase response and viral infection. Immunol Rev. 1992;127:25–50. - PubMed
-
- Alam T, An M R, Mifflin R C, Hsieh C C, Ge X, Papaconstantinou J. Trans-activation of the alpha 1-acid glycoprotein gene acute phase responsive element by multiple isoforms of C/EBP and glucocorticoid receptor. J Biol Chem. 1993;268:15681–15688. - PubMed
-
- Alam T, An M R, Papaconstantinou J. Differential expression of three C/EBP isoforms in multiple tissues during the acute phase response. J Biol Chem. 1992;267:5021–5024. - PubMed
-
- Allen M C. An overview of long-term outcome. In: Witter F R, Keith L G, editors. Textbook of prematurity: antecedents, treatment, and outcome. Boston, Mass: Little, Brown & Co.; 1993. pp. 371–384.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
