The leukemic protein core binding factor beta (CBFbeta)-smooth-muscle myosin heavy chain sequesters CBFalpha2 into cytoskeletal filaments and aggregates
- PMID: 9819429
- PMCID: PMC109324
- DOI: 10.1128/MCB.18.12.7432
The leukemic protein core binding factor beta (CBFbeta)-smooth-muscle myosin heavy chain sequesters CBFalpha2 into cytoskeletal filaments and aggregates
Abstract
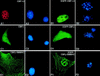
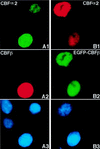
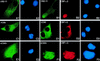
The fusion gene CBFB-MYH11 is generated by the chromosome 16 inversion associated with acute myeloid leukemias. This gene encodes a chimeric protein involving the core binding factor beta (CBFbeta) and the smooth-muscle myosin heavy chain (SMMHC). Mouse model studies suggest that this chimeric protein CBFbeta-SMMHC dominantly suppresses the function of CBF, a heterodimeric transcription factor composed of DNA binding subunits (CBFalpha1 to 3) and a non-DNA binding subunit (CBFbeta). This dominant suppression results in the blockage of hematopoiesis in mice and presumably contributes to leukemogenesis. We used transient-transfection assays, in combination with immunofluorescence and green fluorescent protein-tagged proteins, to monitor subcellular localization of CBFbeta-SMMHC, CBFbeta, and CBFalpha2 (also known as AML1 or PEBP2alphaB). When expressed individually, CBFalpha2 was located in the nuclei of transfected cells, whereas CBFbeta was distributed throughout the cell. On the other hand, CBFbeta-SMMHC formed filament-like structures that colocalized with actin filaments. Upon cotransfection, CBFalpha2 was able to drive localization of CBFbeta into the nucleus in a dose-dependent manner. In contrast, CBFalpha2 colocalized with CBFbeta-SMMHC along the filaments instead of localizing to the nucleus. Deletion of the CBFalpha-interacting domain within CBFbeta-SMMHC abolished this CBFalpha2 sequestration, whereas truncation of the C-terminal-end SMMHC domain led to nuclear localization of CBFbeta-SMMHC when coexpressed with CBFalpha2. CBFalpha2 sequestration by CBFbeta-SMMHC was further confirmed in vivo in a knock-in mouse model. These observations suggest that CBFbeta-SMMHC plays a dominant negative role by sequestering CBFalpha2 into cytoskeletal filaments and aggregates, thereby disrupting CBFalpha2-mediated regulation of gene expression.
Figures
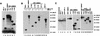




References
-
- Adya, N., and P. P. Liu. Unpublished data.
-
- Arthur D C, Bloomfield C D. Association of partial deletion of the long arm of chromosome 16 and bone marrow eosinophilia in acute non-lymphocytic leukemia. Blood. 1983;62:931. . (Letter.) - PubMed
-
- Bae S C, Yamaguchi-Iwai Y, Ogawa E, Maruyama M, Inuzuka M, Kagoshima H, Shigesada K, Satake M, Ito Y. Isolation of PEBP2 α B cDNA representing the mouse homolog of human acute myeloid leukemia gene, AML1. Oncogene. 1993;8:809–814. - PubMed
-
- Cameron S, Taylor D S, TePas E C, Speck N A, Mathey-Prevot B. Identification of a critical regulatory site in the human interleukin-3 promoter by in vivo footprinting. Blood. 1994;83:2851–2859. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
