Signaling from polyomavirus middle T and small T defines different roles for protein phosphatase 2A
- PMID: 9819441
- PMCID: PMC109336
- DOI: 10.1128/MCB.18.12.7556
Signaling from polyomavirus middle T and small T defines different roles for protein phosphatase 2A
Abstract
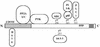
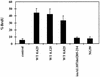
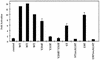
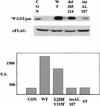
Polyomavirus causes a broad spectrum of tumors as the result of the action of its early proteins. This work compares signaling from middle T antigen (MT), the major transforming protein, to that from small T antigen (ST). The abilities of MT mutants to promote cell cycle progression in serum-starved NIH 3T3 cells were compared. Transformation-defective mutants lacking association with SHC or with phosphatidylinositol 3-kinase (PI3-K) retained the ability to induce DNA synthesis as measured by bromodeoxyuridine incorporation. Only when both interactions were lost in the Y250F/Y315F double mutant was MT inactive. ST promoted cell cycle progression in a manner dependent on its binding of protein phosphatase 2A (PP2A). Since the Y250F/Y315F MT mutant was wild type for PP2A binding yet unable to promote cell cycle progression, while ST was capable of promoting cell cycle progression, these experiments revealed a functional difference in MT and ST signaling via PP2A. Assays testing the abilities of MT and ST to induce the c-fos promoter and to activate c-jun kinase led to the same conclusion. ST, but not Y250F/Y315F MT, was able to activate the c-fos promoter through its interaction with PP2A. In contrast, MT, but not ST, was able to activate c-jun kinase by virtue of its interaction with PP2A.
Figures
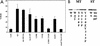
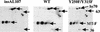
References
-
- Asselin C, Sullivan M, Bastin M. Introns enable the polyomavirus middle and small T antigens to stimulate the growth of primary rat embryo fibroblasts. Gene. 1997;203:175–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
